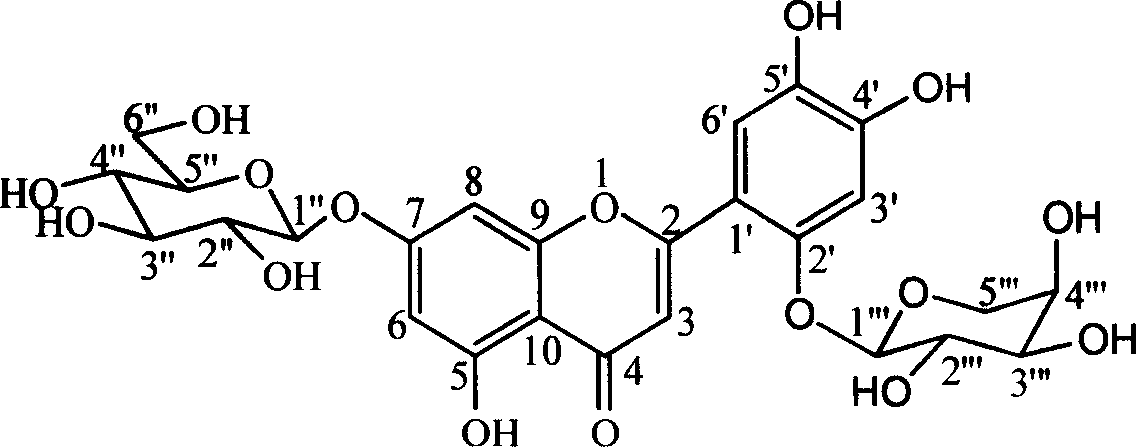
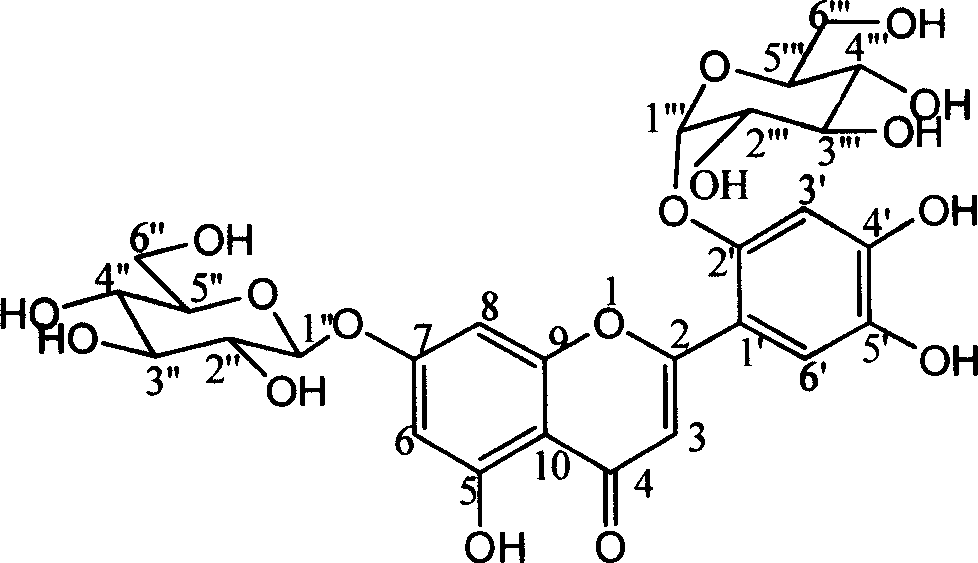
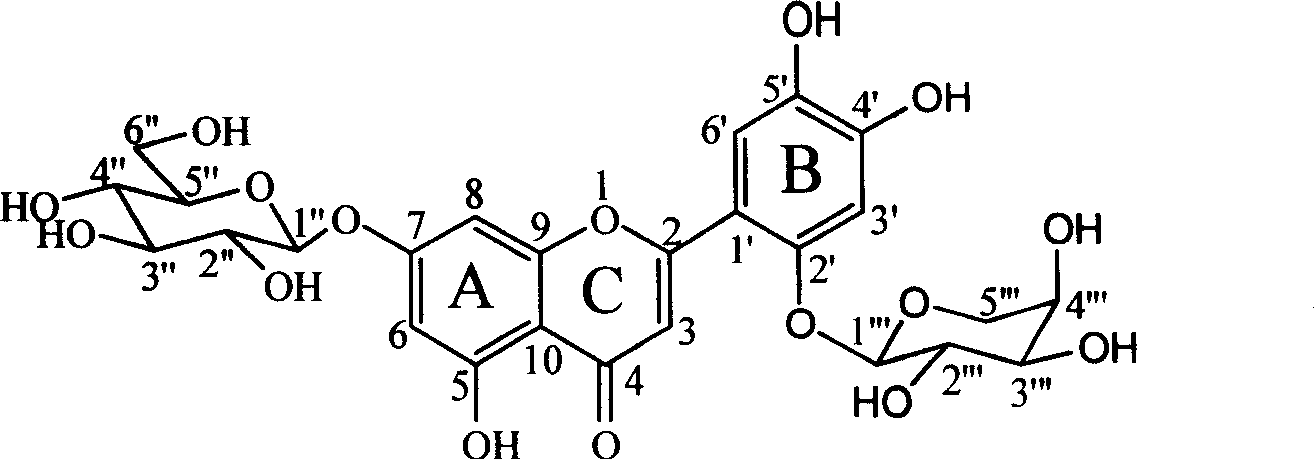
Two flavone glycosides in dandelion and medical use for against Gram-positive bacterium thereof
A technology of dandelion glycosides and dandelion, which is applied in the field of medicine and can solve problems such as unclear chemical components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Separation of Mongolian Taraxacin A and Mongolian Taraxacin B:
[0024] 1.1 Instruments and reagents
[0025]The melting point was measured with a micro melting point apparatus (produced by Beijing Tektronix Co., Ltd.), and the temperature was not corrected; the optical rotation was measured on a Polax-2L automatic polarimeter produced in Japan; the infrared spectrum (IR) was measured by a Bruker Vector-22 infrared spectrometer, and the KBr tablet ; Ultraviolet spectrum is measured with Shimadzu UV-240 ultraviolet spectrophotometer; Proton nuclear magnetic resonance spectrum ( 1 H-NMR), carbon nuclear magnetic resonance spectrum ( 13 C-NMR) and two-dimensional nuclear magnetic resonance (2D NMR) were determined by INOVA superconducting nuclear magnetic resonance spectrometer (VARIAN INOVA-400 MHz) (tetramethylsilyl ether TMS as internal standard); electrospray mass spectrometry (ESI-MS ) was measured by Bruker Esquire 3000+ mass spectrometer, silica gel GF...
Embodiment 2
[0048] Example 2: Detection of the Ability of Flavonoid Glycosides 1 and 2 to Inhibit Gram-positive Bacteria
[0049] 2.1 Principle:
[0050] The "tube-and-disk method" is used to study the in vitro antibacterial activity of the test drug, which is to use the test drug added in the Oxford cup to diffuse into the medium inoculated with the test bacteria, thereby inhibiting the growth of the bacteria. The diameter of the bacterial circle was used to determine the antibacterial activity of the test drug.
[0051] 2.2 Detection of bacteria:
[0052] Staphylococcus aureus 26003-23 was purchased from China Institute for the Control of Pharmaceutical and Biological Products.
[0053] Beta-hemolytic Streptococcus 32210 was purchased from China Institute for the Control of Pharmaceutical and Biological Products.
[0054] 2.3 Test drugs:
[0055] DMSO: DMSO solvent control;
[0056] Norfloxacin: 1.25mg / ml;
[0057] Mongolian taraxacin A (compound 1): 5.0mg / ml; Mongolian taraxa...
Embodiment 3
[0074] Example 3: Comparison of Compounds 1 and 2 in Rhizomes and Shoots and Leaves of Five Species of Dandelion
[0075] Dandelion (Mongolian dandelion) (Taraxacum mongolicum Hand-Mazz), Alkaline dandelion (Hua dandelion) (Taraxacum sinicum Kitag), Jehol dandelion (White margin dandelion) (Taraxacum platypecidum Diels), Northeast dandelion (Taraxacum ohwianum Kitag), anti-bract dandelion (Taraxacum grypodon Dahlst) and Xing'an dandelion (Taraxacum falcilobum Kitag) each 20 grams were collected and identified by Professor Peng Hua from Kunming Institute of Botany, Chinese Academy of Sciences.
[0076] Waters high performance liquid chromatography (Waters HPLC, 2695 separation system comprises quaternary pump, column thermostat autosampler; 2996 ultraviolet diode matrix detector, Empower chromatographic operation software and Waters Symmetry RP-18 4.6mm×250mm, 5μm chromatographic column) was used to detect the content of compounds 1 and 2.
[0077] Treatment of test sample...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com