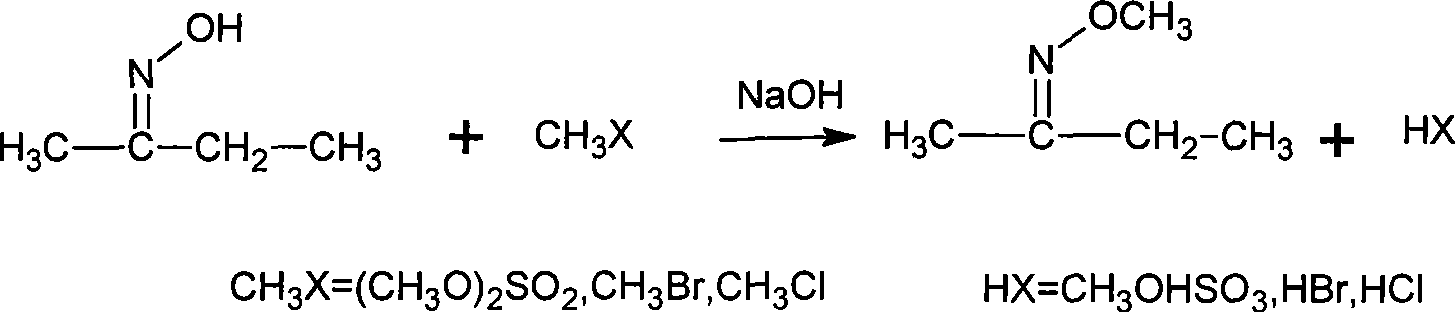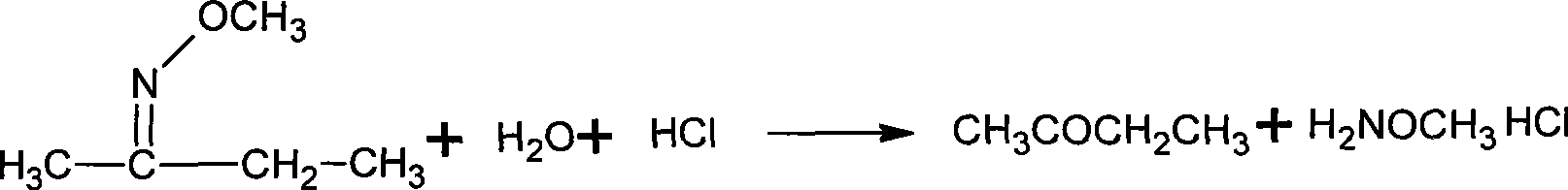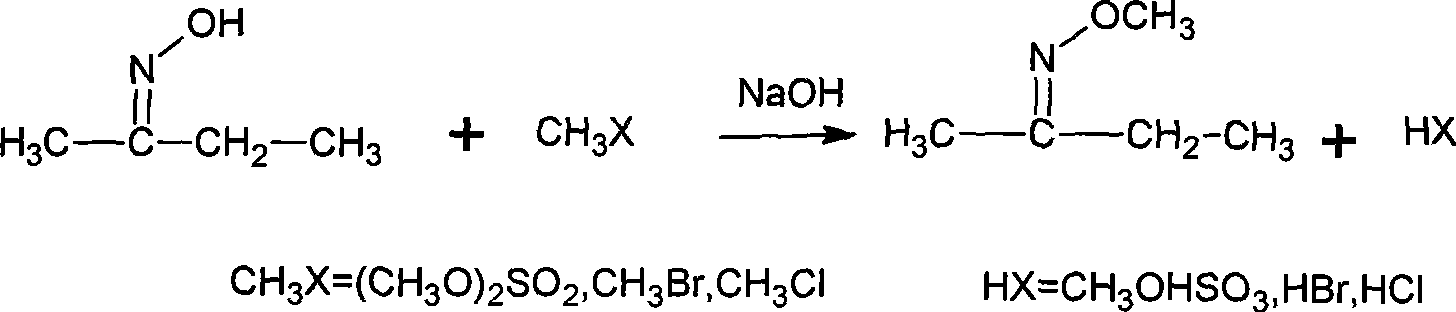Method for synthesizing methoxy amine hydrochlorate
A technology of methoxyamine hydrochloride and methyl, which is applied in the field of compound synthesis, can solve the problems of high toxicity, pollution, yield drop, and high recycling cost, and achieve the effects of reducing exhaust gas emissions, mild reaction conditions, and reducing investment costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 90g of water, 10g (0.25mol) NaOH into a 150mL flask equipped with electric stirring, thermometer, and condenser, stir and dissolve at room temperature, then 8.7g (0.10mol) butanone oxime, 0.3g PEG500, and then cool to 5°C , began to add 16.4g (0.13mol) of dimethyl sulfate, added for 1h, and reacted at 10°C for 4h after the addition was completed. Then cool to below 5°C and let it stand for more than 1 hour, then separate the upper organic layer, that is, the oil layer, to obtain 9.0g; the water layer is heated up and distilled, and the distillate is collected, and the distillation temperature ends when the gas phase temperature reaches 95°C, to obtain 22.3g. Combine the oil layer and distillate, add 22.6g of 30% hydrochloric acid (0.19mol) and mix well.
[0035] Prepare a glass rectification column with an inner diameter of 20mm and a height of 1600mm equipped with spring glass packing. The rectification column is divided into two parts with the same height. There i...
Embodiment 2
[0037] The amount of NaOH was changed to 0.20 mol, and the others were the same as in Example 1. The result was 6.5 g of methoxylamine hydrochloride, the yield was 77.8%, and the product mole fraction was 98.8%.
Embodiment 3
[0039] The water consumption was changed to 45g, and the others were the same as in Example 1. The result was 6.3g of methoxylamine hydrochloride, a yield of 75.4%, and a product mole fraction of 98.7%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com