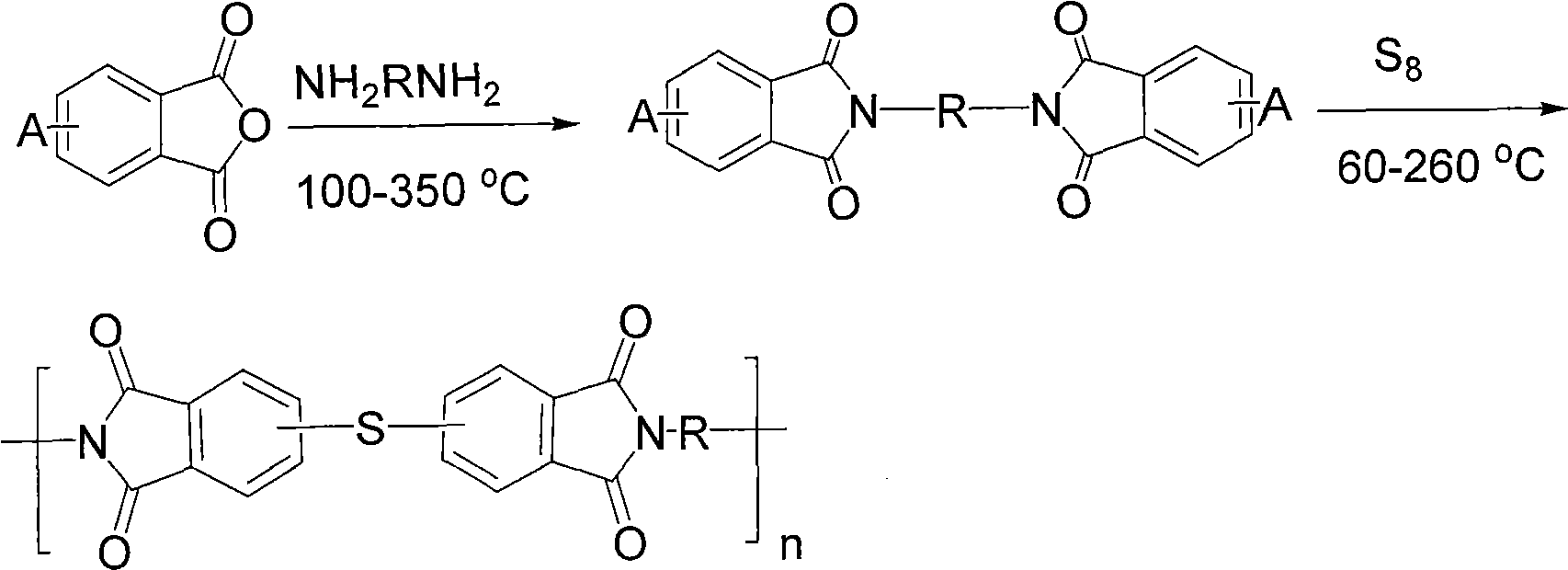
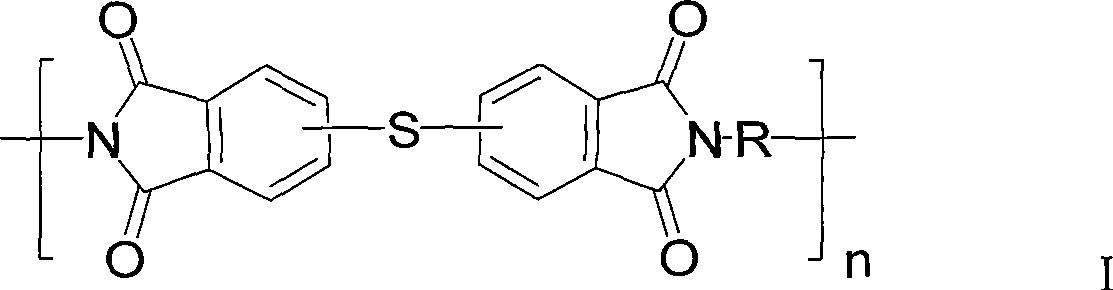
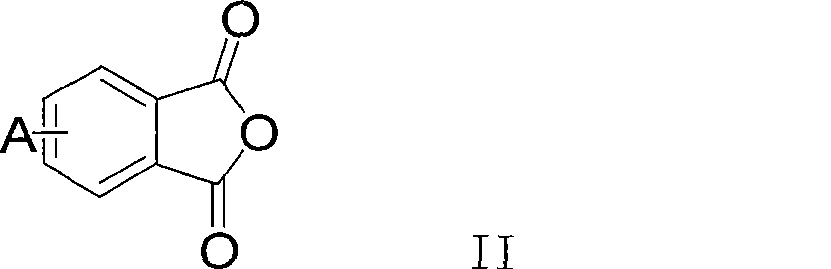Polythioetherimide and preparation method thereof
A technology of polythioetherimide and phthalimide, which is applied in the field of preparation of new polythioetherimide, which can solve the problem of difficulty in controlling the equimolar equivalent of dichloromonomers and the easy oxidation and deliquescence of anhydrous alkali metal sulfides , high requirements for preparation conditions, etc., to achieve the effects of saving preparation costs, simple and easy operation and production, and reasonable and practical technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 32.86g (0.18mol) 4-chlorophthalic anhydride, 3.65g (0.02mol) 3-chlorophthalic anhydride, 400mL glacial acetic acid into a dry and clean 1L three-necked flask, stir to dissolve and add 19.83g (0.1mol) 4,4 '-Diaminodiphenylmethane, heated to 140°C for reflux reaction for 24 hours, cooled to room temperature and filtered the obtained filter cake, washed three times with distilled water, and vacuum-dried at 120°C to obtain 48.52g of crude product of dichlorophthalimide, yield 92%. The crude product was recrystallized from a mixed solvent of toluene and N,N-dimethylacetamide (4:1, v / v) and used for the next step of polymerization. Under an argon atmosphere, add 7.9107g (0.015mol) of the above dichloromonomer, 0.4800g (0.015mol) of sulfur, 1.3241g (0.035mol) of sodium borohydride, 1.7954g (0.032mol) of hydrogen hydride into a dry and clean 500mL three-necked flask Potassium oxide, 0.4439g (0.004mol) calcium chloride and 150mL N,N-dimethylacetamide, stirred and heated to ...
Embodiment 2
[0036] Add 91.28g (0.50mol) 4-chlorophthalic anhydride, 91.28g (0.50mol) 3-chlorophthalic anhydride, 100.12g (0.5mol) 4,4'-diaminodiphenyl ether into a dry and clean 500mL three-necked flask, Slowly heated up to 260°C under vacuum to form a homogeneous melt, stirred and reacted for 4 hours, and then cooled to room temperature to obtain 259.38 g of crude bischlorophthalimide, with a yield of 98%. The crude product can be directly used in the next polymerization reaction. In a dry and clean 500mL three-necked flask, add 10.5872g (0.020mol) of the above dichloro monomer, 0.6602g (0.0206mol) of sulfur, 0.9600g (0.04mol) of sodium hydride, 2.8471g (0.0206mol) of potassium carbonate, 0.2120g ( 0.005mol) lithium chloride and 180mL N-methylpyrrolidone, stirred and heated to 80°C for 24 hours, then added 0.3092g (0.0012mol) N-phenyl-3-chlorophthalimide and reacted for 4 hours After cooling to room temperature, the reaction solution was slowly poured into 2L distilled water and stirred...
Embodiment 3
[0038] Add 109.54g (0.60mol) 4-chlorophthalic anhydride, 36.51g (0.20mol) 3-chlorophthalic anhydride, 1600mL glacial acetic acid into a dry and clean 3L three-necked flask, stir to dissolve and add 90.53g (0.40mol) 3,3 '-Dimethyl-4,4'-diaminodiphenylmethane, heat up to 130°C and reflux for 30 hours, cool to room temperature, pour it into 6L distilled water and stir for 3 hours, filter the filter cake and wash it with distilled water Three times, vacuum drying at 120° C. yielded 208.84 g of a crude product of dichlorophthalimide, with a yield of 94%. The crude product was recrystallized from dimethyl sulfoxide and used in the next polymerization reaction. Under a nitrogen atmosphere, add 2.7772g (0.005mol) of the above-mentioned dichloromonomer, 0.1600g (0.005mol) of sulfur, 0.4504g (0.005mol) of paraformaldehyde, 0.5299g (0.005mol) of carbonic acid into a dry and clean 100mL three-necked flask Sodium, 0.0530g (0.0012mol) lithium chloride, 50mL hexamethylphosphoric triamide, s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com