Method for preparing D-cystine and L-tryptophane by using DL-cysteine split by microbial enzyme method
A cysteine, microbial enzyme technology, applied in microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve problems such as enantiomer consumption, achieve good temperature tolerance, high catalytic efficiency, The effect of good industrial value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Construction of Genetic Engineering Bacillus subtilis and High Expression of Tryptophanase
[0035] According to the tryptophanase gene sequence of Escherichia coli K12 strain, design the upstream primer P1 of the tryptophanase gene: 5'-CCG AAGCTT ATG GAA AAC TTT AAA CAT CTC C-3' and the downstream primer P2: 5'-CCC GGA TCC TFA AAC TFCTTT CAG TTT TGC GG-3', using the genomic DNA of Escherichia coli JM109 strain as a template, the tryptophanase gene was amplified by PCR, and the recombinant expression plasmid pWB980-tnaA was constructed respectively.
[0036] The recombinant plasmid was transformed into Bacillus subtilis WB600, and a tryptophanase genetically engineered strain was constructed.
[0037] Inoculate the positive recombinant strain into 4 mL of LB medium containing 20 μg / mL of kanamycin for overnight culture, then inoculate 50 mL of fresh LB medium containing 20 μg / mL of kanamycin at 37°C Shake culture for 24 hours, remove the bacteria by centrifugation, tak...
Embodiment 2
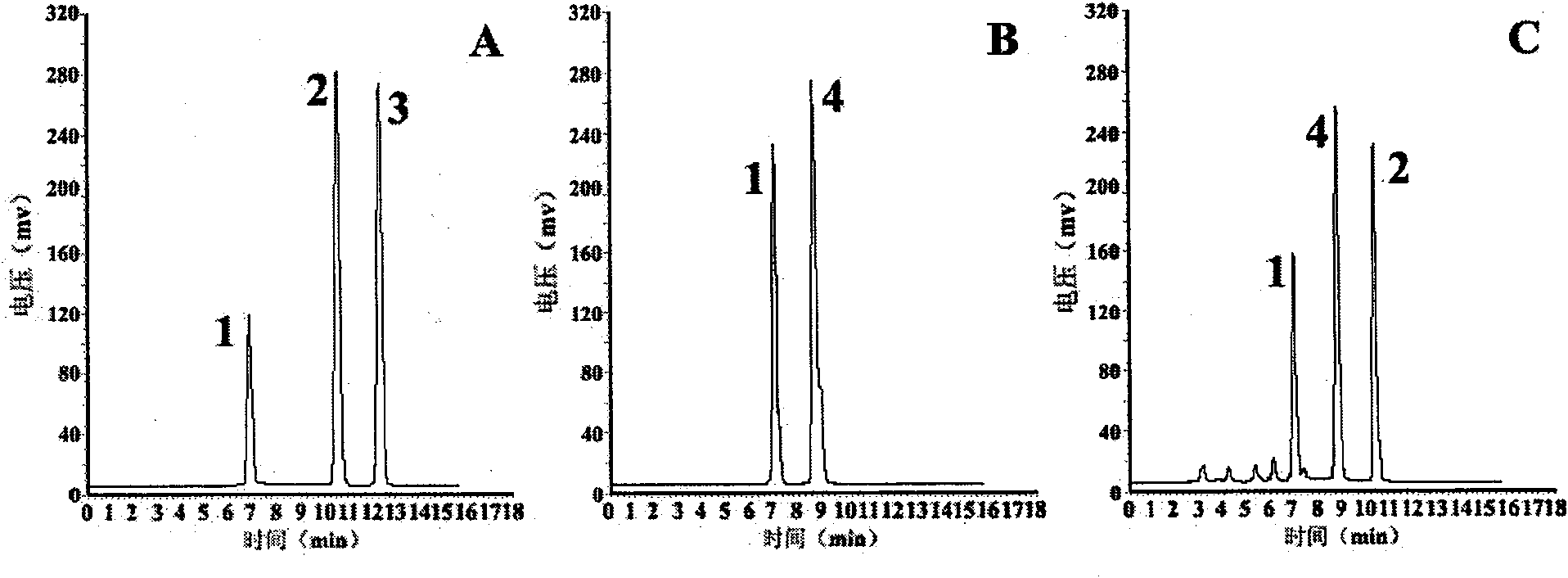
[0039] Determination of D / L-cysteine by pre-column derivatization HPLC
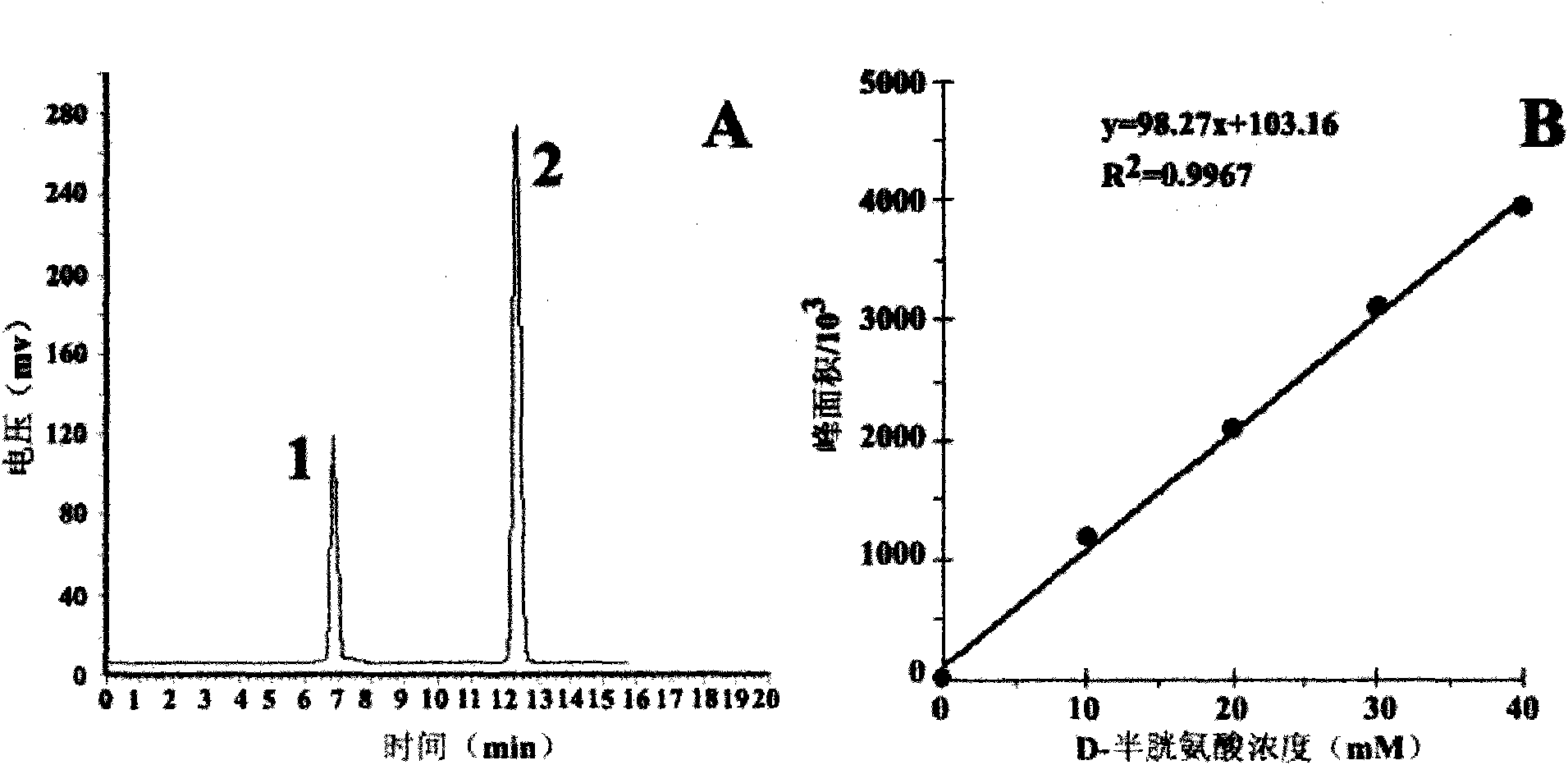
[0040] The pre-column derivatization method is as follows: with 100μL 10mmol / L Na 2 CO 3 solution (pH9) to dissolve cysteine to a final concentration of 10-40mmol / L, then add 1% FDAA 200μL acetone solution, incubate at 40°C for 1h, add 2N HCl 20μL to terminate the reaction, and inject 20μL for HPLC analysis. Chromatographic conditions: C 18 Chromatographic column (Phenomenex Luna 5μ, 100A, 250×4.6mm), using phase A water (containing 0.1% TFA) and phase B acetonitrile (containing 0.1% TFA) as mobile phases, gradient elution: 0.0min, 55% phase A ; 11.0min, 47% phase A; room temperature, detection wavelength 340nm, flow rate 1mL / min. Inject 5 times continuously, calculate the peak area and take the average value, draw the standard curve with the D-cysteine concentration (X, mmol / L) as the abscissa and the peak area (Y) as the ordinate. The D-cysteine produced by the enzymatic reaction was dilute...
Embodiment 3
[0042] Determination of L-Tryptophan Content by HPLC
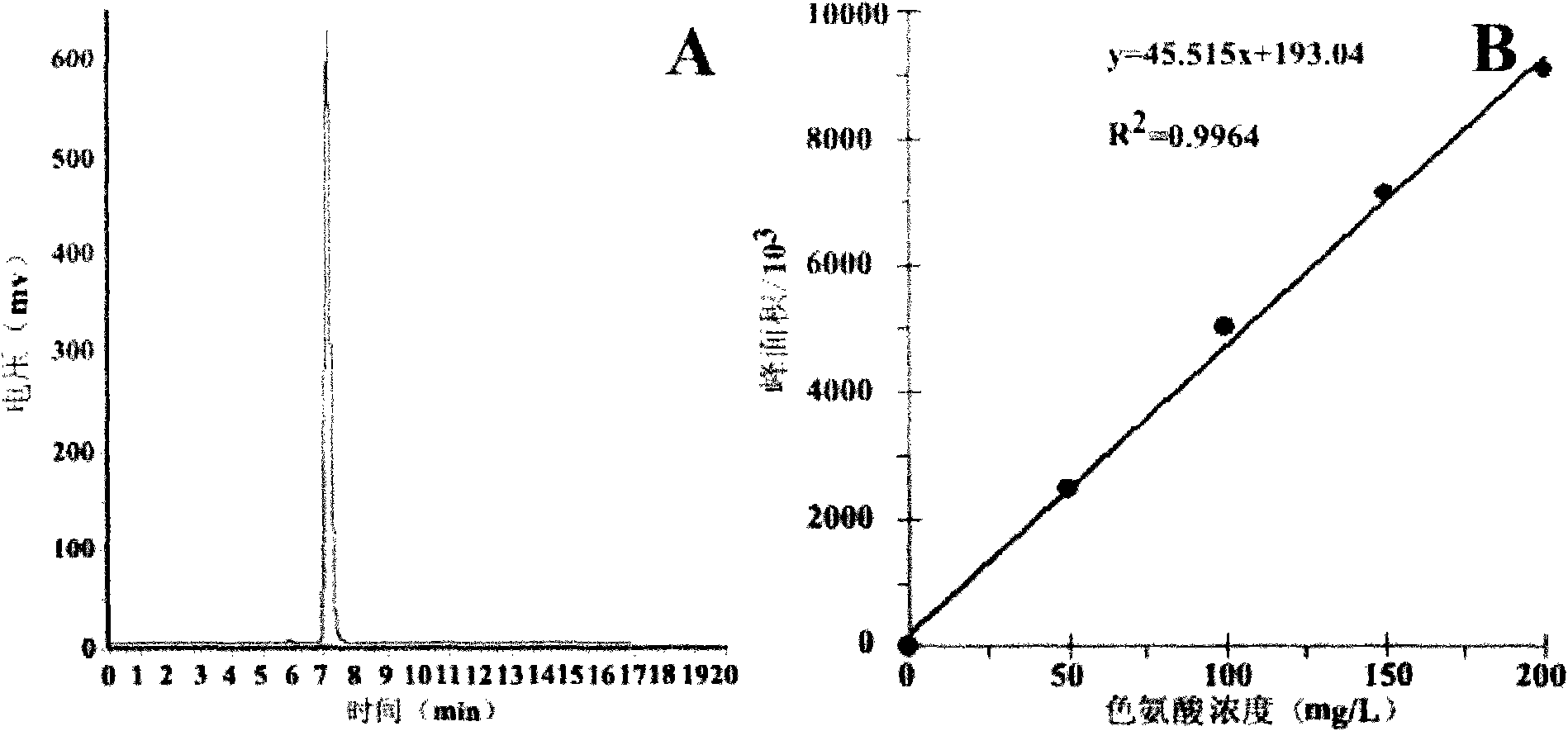
[0043] Precisely configure L-tryptophan standard solution with a concentration of 50-200 mg / L, take 20 μL of samples for injection, and use HPLC method to determine the content of L-tryptophan. Chromatographic conditions: C18 chromatographic column (Phenomenex Luna 5μ, 100A, 250×4.6mm), mobile phase: methanol: 0.001mol / L potassium dihydrogen phosphate (30:70), room temperature, detection wavelength 225nm, flow rate 1mL / min. Inject 5 times continuously, calculate the peak area and take the average value, draw the standard curve with the L-tryptophan concentration (X, mg / L) as the abscissa and the peak area (Y) as the ordinate.
[0044] The L-tryptophan produced by the enzymatic reaction and the refined L-tryptophan can be accurately quantified after being detected by high performance liquid chromatography and compared with the standard curve. Figure 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com