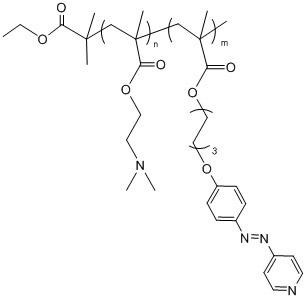
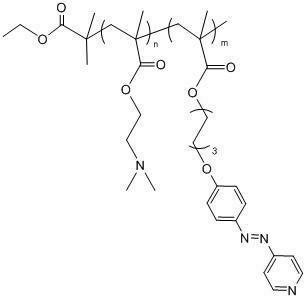
Method for preparing multi-responsive block copolymer containing azopyridine
A technology of block copolymer and azopyridine, which is applied in the field of preparation of multi-responsive block copolymer, and achieves the effect of simple and easy synthesis method and wide sources.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Dissolve 5 g of phenol and 4 g of sodium nitrite in 20 mL of 10% aqueous sodium hydroxide solution, and 6 g of 4-aminopyridine in 45 mL of 7.3 mol / L hydrochloric acid solution. At 0 °C, the former solution was added dropwise to the latter, after mixing, the pH value of the mixed solution was adjusted to 6 with 10% NaOH solution, filtered, and the precipitate was purified and dried to obtain product A. Then, 4 g of product A, 5.5 g of potassium carbonate, 3 g of 6-chloro-1-hexanol, and 5 g of potassium iodide were dissolved in an appropriate amount of solvent, reacted at 70 °C for 8 hours, purified and dried to obtain product B. Dissolve 3 g of product B in tetrahydrofuran, add 1.55 mL of triethylamine, then dropwise add 1 mL of methacryloyl chloride solution, react for 7 hours, pour the reaction solution into water to obtain a precipitate, and pass the dried precipitate through column chromatography After that, the photoresponsive monomer is obtained.
[0026] Dissolve...
Embodiment 2
[0028] Dissolve 5 g of phenol and 4 g of sodium nitrite in 25 mL of 10% aqueous sodium hydroxide solution, and 6 g of 2-aminopyridine in 55 mL of 7.3 mol / L hydrochloric acid solution. At -5 °C, the former solution was added dropwise to the latter, after mixing, the pH value of the mixed solution was adjusted to 7 with 10% NaOH solution, filtered, and the precipitate was purified and dried to obtain product A. Dissolve 4 g product A, 5.5 g potassium carbonate, 3 g 6-chloro-1-hexanol, and 6 g potassium iodide in an appropriate amount of dimethylformamide solvent, react at 85 °C for 10 hours, purify and dry to obtain Product B. Dissolve 3 g of product B in chloroform, add 2 mL of diethylamine, and then dropwise add it to a solution of 1.2 mL of methacryloyl chloride. After reacting for 9 hours, pour the reaction solution into water to obtain a precipitate. After drying, The photoresponsive monomer was obtained after the precipitate was subjected to column chromatography.
[002...
Embodiment 3
[0031] Dissolve 5 g of phenol and 4 g of sodium nitrite in 25 mL of 10% aqueous sodium hydroxide solution, and 6 g of 3-aminopyridine in 55 mL of 7.3 mol / L hydrochloric acid solution. At 5 °C, the former solution was added dropwise to the latter, after mixing, the pH of the mixed solution was adjusted to 6.5 with 10% NaOH solution, filtered, and the precipitate was purified and dried to obtain product A. Dissolve 4 g product A, 5.5 g potassium carbonate, 3.5 g 6-bromo-1-hexanol, and 6 g potassium iodide in an appropriate amount of dimethyl sulfoxide solvent, react at 95 °C for 12 hours, purify and dry to obtain Product B. Dissolve 3 g of product B in dichloromethane, add 2 mL of diethylamine, and then dropwise add it to a solution of 1.2 mL of methacryloyl chloride. After reacting for 8 hours, pour the reaction solution into water to obtain a precipitate. After drying, The photoresponsive monomer was obtained after the precipitate was subjected to column chromatography.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com