Cyclic lipopeptide antibiotic and preparation and application thereof
A cyclolipopeptide and antibiotic technology, applied in the field of biomedicine, can solve the problems of high price, no oral dosage form, and cannot be administered alone, and achieve the effects of excellent toxicity, obvious prevention and treatment effect, and high medical application value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of cyclolipopeptide antibiotic cyclononapeptide
[0033] (1) Transfer strain B7 from a glycerol tube stored in a -80°C refrigerator to nutrient agar medium, and culture at 32°C for 24-72 hours;
[0034] (2) Put the activated strain into 50mL seed culture medium in a 250mL Erlenmeyer flask, shake and culture at 32°C and 220rpm for 24h, which is the first-grade seed solution;
[0035] (3) Put the first-grade seed solution into 200mL seed medium in a 500mL Erlenmeyer flask, and culture it at 32°C and 220rpm for 24 hours with shaking to obtain the second-grade seed solution;
[0036] (4) Put the secondary seed liquid into a 2L Erlenmeyer flask containing 500mL of fermentation medium at an inoculum amount of 5%-10% (v / v), and culture at 28°C and 200rpm for 96h with shaking to obtain a fermentation broth.
[0037] Nutrient agar medium, prepared as follows: beef extract 3g, peptone 10g, sodium chloride 5g, agar 17g, distilled water 1000mL, pH7.2;
[0038...
Embodiment 2
[0044] Example 2 Determination of cyclolipopeptide antibiotic cyclononapeptide
[0045] The product prepared in Example 1 The relevant parameters of the compound cyclolipopeptide antibiotic cyclononapeptide are as follows:
[0046] Appearance: white powder after freeze-drying;
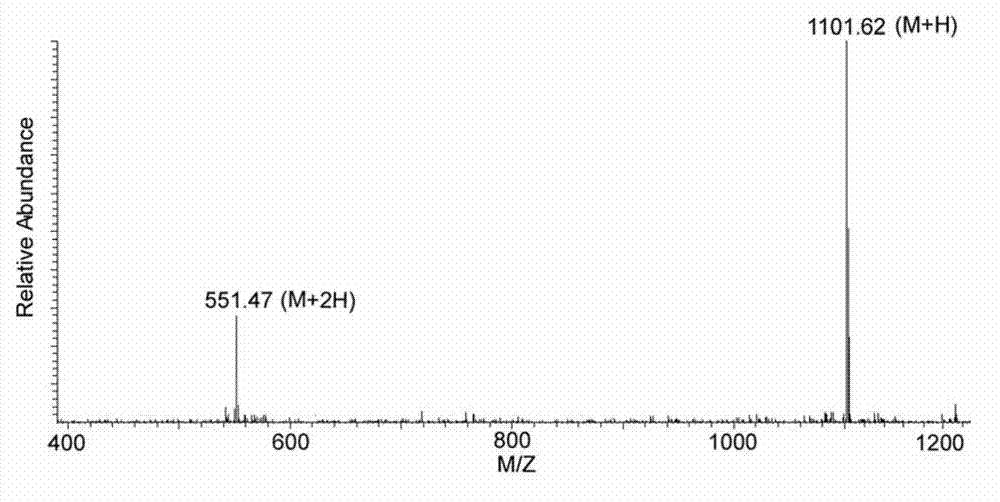
[0047] Molecular weight: 1100;
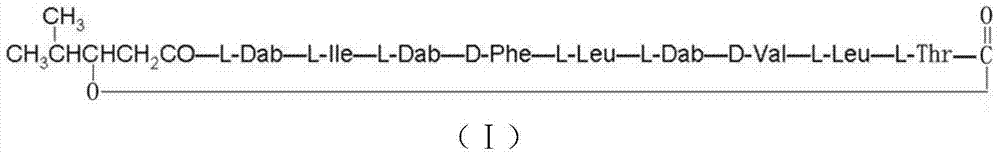
[0048] Molecular formula: C 54 h 92 N 12 o 12 ;
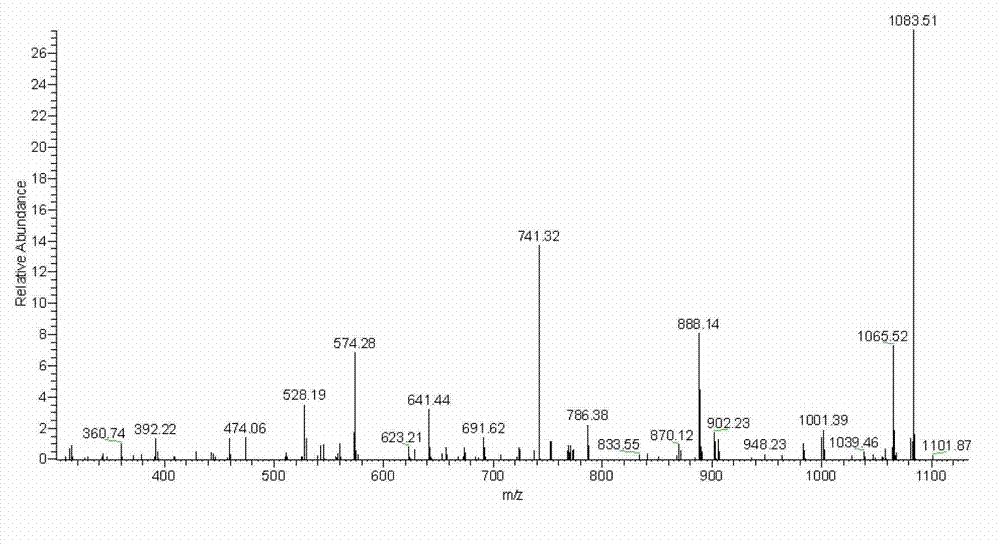
[0049] ESI-MS spectrum: see figure 1 ;
[0050] ESI-MS-MS spectrum: see figure 2 .
[0051] According to the above characteristics, it can be inferred that the compound is a new cyclolipopeptide antibiotic containing nine amino acids, and it is named cyclononapeptide. The antibiotic is another new cyclolipopeptide antibiotic isolated from Paenibacillus for the first time at home and abroad. The compound showed significant inhibitory activity against all tested Gram-positive (staphylococcus) and negative (pseudomonas and Escherichia coli) pathogenic bacteria and fungi (Candida albicans). Experiments in mice s...
Embodiment 3
[0052] Example 3 Determination of in vitro antibacterial activity of cyclolipopeptide antibiotic cyclononapeptide
[0053] Use Mueller Hinton broth medium to prepare cyclic nonapeptide antibiotics at a concentration of 256 μg / mL, and adjust the concentration of cyclic nonapeptides to 256, 128, 64, 32, 16, 8, 4, 2, 1, 0.5 μg / mL.
[0054] Add 50 μL of doubly diluted cyclic nonapeptide antibiotic solution to the 1st to 10th wells of a 96-well polystyrene plate, and add 50 μL of Mueller Hinton broth medium (acid hydrolyzed casein 17.5 g / L) to the 11th well , beef extract powder 2g / L, starch 1.5g / L) as a growth control, and 100 μL of Mueller Hinton broth medium was added to the 12th well as a negative control. The control antibiotic was polymyxin B.
[0055] After culturing the indicator bacteria for 24 hours, dilute to 10 with Mueller Hinton broth medium 6 cells / mL, add 50 μL of diluted bacterial solution to the 1st to 11th wells, seal and incubate in an incubator at 37°C for 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com