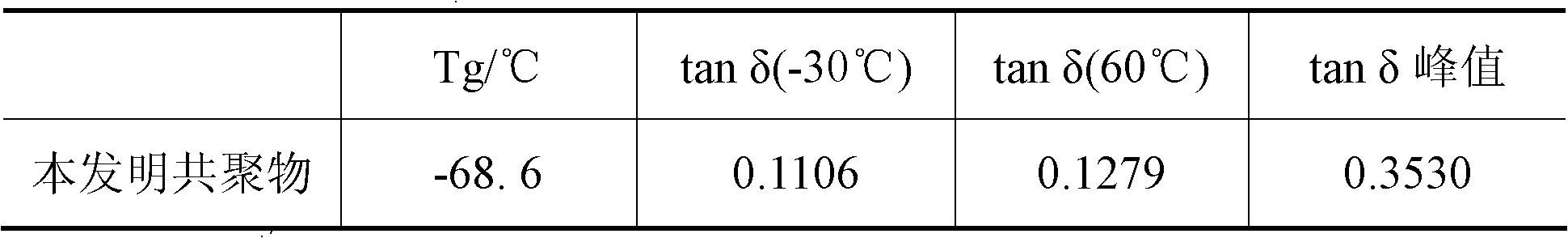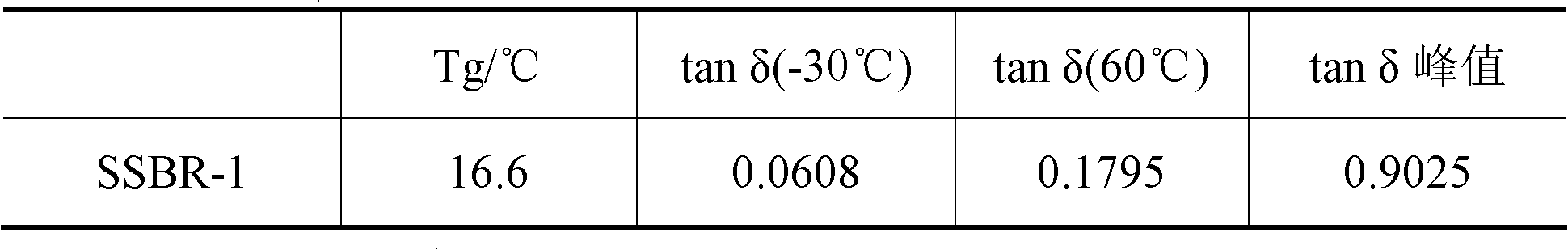Stereo-regular conjugated diene/styrene copolymer and preparation method thereof
A technology of styrene copolymer and conjugated diene, which is applied in the field of stereoregular conjugated diene/styrene copolymer and its preparation, and can solve problems such as low tear strength, increased molecular chain friction, and large hysteresis loss. problems, to achieve the effect of low glass transition temperature, strong resistance to deformation, energy saving and consumption reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] At -10°C under nitrogen protection, 1.0mmol Al(i-Bu) 3 (B), 0.1mmol AlH(i-Bu) 2 (B), a mixture of 1.2mmol chloroform (C) and 0.1mmol ethyl trichloroacetate (D) was mixed with 0.06mmol neodymium octanoate (A), reacted for 148 hours to form a homogeneous catalyst, and set aside. Wherein the molar ratio of each component is A:B:C:D=1:16:20:1.5.
[0033] Under nitrogen protection, 10 ml of a mixed solution of styrene (St), butadiene (Bd), hexane and cyclohexane was added to the dry polymerization reactor, wherein Bd / St=1.0 (mass ratio). The above-mentioned homogeneous catalyst was added, and polymerization was carried out at 60° C. for 5 hours. The reaction was terminated with an ethanol solution containing 1% anti-aging agent 1076, and the polymer product was extracted with n-hexane and butanone for 24 hours, washed with water, and dried in a vacuum oven at 40°C until constant weight. M of the resulting copolymer w 4.7×10 5 g / mol, M w / M n 4.3, cis-1, 4 content is 9...
Embodiment 2
[0035] Under the protection of nitrogen at 15°C, mix 0.12mmol of neodymium (A) with 1.9mmol of Al(i-Bu) 3 (B), 0.2mmol AlH(i-Bu) 2 (B), a mixture of 1.0mmol chloroform (C), 0.2mmol trichloroethane (C) and 0.2mmol tert-butyl trichloroacetate (D) was reacted for 75 minutes to form a homogeneous catalyst and set aside. Wherein the molar ratio of each component is A:B:C:D=1:16:10:0.8.
[0036] Add 10 ml of a mixed solution of styrene (St), isoprene (Ip) and cyclohexane into the dry polymerization reactor under the protection of nitrogen, wherein Ip / St=0.6 (mass ratio). The above-mentioned homogeneous catalyst was added, and polymerization was carried out at 70° C. for 5 hours. The molar ratio of catalyst component A to total monomer is 5.3×10 -3 . The termination reaction and post-treatment method are the same as implementation 1. M of the resulting copolymer w 3.8×10 5 g / mol, M w / M n 3.9, cis-1, 4 content is 96.0% (mol), total styrene binding amount is 10wt%, molecular ...
Embodiment 3
[0038] Add 2.4mmol of Al(i-Bu) to the catalyst preparation device at 45°C under nitrogen protection 3 (B), 0.1mmol AlH(i-Bu) 2 (B), then add a mixed solution of 0.2mmol ethyl trichloroacetate (D) and 3.0mmol chloroform (C), then mix with 0.15mmol neodymium octanoate (A), and react for 1.5 hours to form a homogeneous catalyst. spare. Wherein the molar ratio of each component is A:B:C:D=1:18:20:1.4.
[0039] The polymerization method is the same as in Example 1. After 2 hours of polymerization at 40°C, the temperature is raised to 60°C and the polymerization is continued for 3 hours. The molar ratio of catalyst component A to total monomers is 1.5×10 -3 . The termination reaction and post-treatment method are the same as implementation 1. M of the resulting copolymer w 1.0×10 5 g / mol, M w / M n 5.2, cis-1, 4 content is 93.7% (mol), total styrene binding amount is 44.4wt%, PS segment molecular weight is 4×10 4 g / mol, the tacticity (rrrr sequence) is 80%, the PS segment ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mw | aaaaa | aaaaa |
| Mw | aaaaa | aaaaa |
| Mw | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com