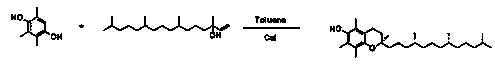
Environmentally-friendly synthesis method of vitamin E
A green and environmentally friendly synthesis method technology, applied in the field of fine chemical synthesis, can solve problems such as complex operation, equipment corrosion, environmental pollution, and high production costs, and achieve the effects of increasing reaction yield, shortening reaction cycle, and improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In the reaction device equipped with a water separator and a reflux tube condenser, add 200mL of toluene, and add 40g (0.263moL, 1.3Eqv) of 2,3,5-trimethylhydroquinone and 66g of isophytol in sequence under mechanical stirring (0.2moL, 1.0Eqv), after the reaction system is fully dissolved, slowly add 2 grams of magnesium chloride and 1 gram of self-made magnesia-supported silica catalyst, close the reaction system, vacuumize and replace with argon three times, and finally vacuumize to ensure the reaction The system is maintained at a vacuum degree of about -0.06MPa, and the temperature is slowly raised to about 60°C, and the reaction condition is maintained for 2 hours. As the reaction progresses, water is continuously distilled out with the reflux of toluene. After the reaction is completed by Gc detection, The self-made supported catalyst was separated by filtration, rinsed with a small amount of toluene, dried for repeated application, collected the toluene phase,...
Embodiment 2
[0042] Preparation of magnesia-supported silica catalyst: Add 100g of magnesium nitrate into 800g of water and stir fully, slowly raise the temperature to 35°C, add sodium carbonate to adjust the pH value of the reaction to 10, then add 95g of 350 mesh silica, and keep the temperature fully Immerse for 4-6 hours, then lower the system to room temperature, filter, dry the solvent, sinter at 350° C. for 2.5 hours, and granulate to obtain a granular magnesia-supported silicon dioxide catalyst.
[0043] Add 200mL of toluene (relative density 0.87g / ml) to the reaction device equipped with a water separator and a reflux tube condenser, and add 40g (0.263moL, 1.0Eqv) and isophytol 77.9g (0.263moL, 1.0Eqv), after the reaction system is fully dissolved, slowly add 2 grams of magnesium chloride and 1 gram of self-made magnesium oxide-supported silica catalyst, seal the reaction system, vacuumize with argon The gas was replaced three times, and finally vacuumed to ensure that the reactio...
Embodiment 3
[0045] Preparation of magnesia-supported silica catalyst: Add 100g of magnesium nitrate into 1000g of water and stir fully, slowly raise the temperature to 45°C, add sodium carbonate to adjust the pH value of the reaction to 11.5, then add 105g of 450 mesh silica, and keep the temperature fully Immerse for 6 hours, then lower the system to room temperature, filter, dry the solvent, sinter at 450° C. for 3.5 hours, and granulate to obtain a granular magnesia-supported silica catalyst.
[0046] In the reaction device equipped with a water separator and a reflux tube condenser, add 200mL of toluene, and add 40g (0.263moL, 1.6Eqv) of 2,3,5-trimethylhydroquinone and isophytol 48.7 g (0.164moL, 1.0Eqv), after the reaction system is fully dissolved, slowly add 2 grams of magnesium chloride and 1 gram of self-made magnesia-supported silica catalyst, seal the reaction system, vacuumize and replace with argon three times, and finally vacuumize to ensure The reaction system is maintained...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com