Acid-sensitive amphiphilic star-shaped block copolymer as well as preparation method and application thereof
A technology of block copolymer and amphiphilic block, which is applied in the field of acid-sensitive amphiphilic star-shaped block copolymer, can solve the problems of difficult to precisely control polymer structure and molecular weight distribution, low reaction efficiency and so on.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
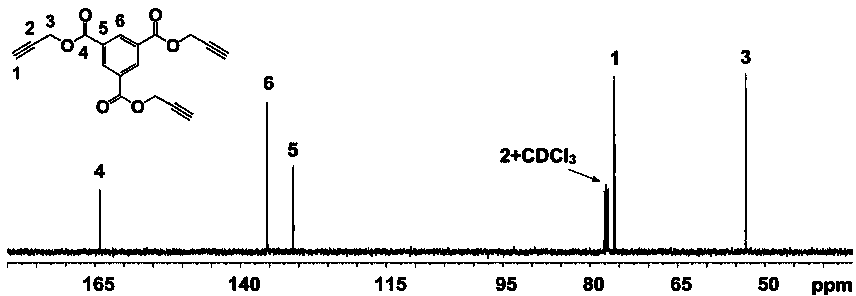
[0081] Example 1: Preparation of Trimellitic Acid Propargyl (TAPE)
[0082] Put the branch tube bottle and the ground glass stopper equipped with a stirrer in an oven at 120°C to dry for at least 24 hours, take it out, put the ground glass stopper on the branch tube bottle stopper and connect it to the double-row tube, and cool it to room temperature with an oil pump. Repeat pumping and inflation three times, and finally fill with argon. Under the condition of argon, tetrahydrofuran (THF, 50 mL), propargyl alcohol (PA, 0.5000 g, 8.92 mmol) and 4-dimethylaminopyridine ( DMAP, 0.9078 g, 7.44 mmol), stirred until the solution was clear and transparent, quickly weighed trimesoyl chloride (BTT, 0.6577 g, 2.48 mmol) and added it to a dropping funnel filled with tetrahydrofuran (10 mL), and kept in an ice-water bath Slowly add it dropwise to the mixture, put the reaction bottle into an oil bath set at 25°C, and react under stirring for 12 hours.
[0083] After the reaction, the prod...
Embodiment 2
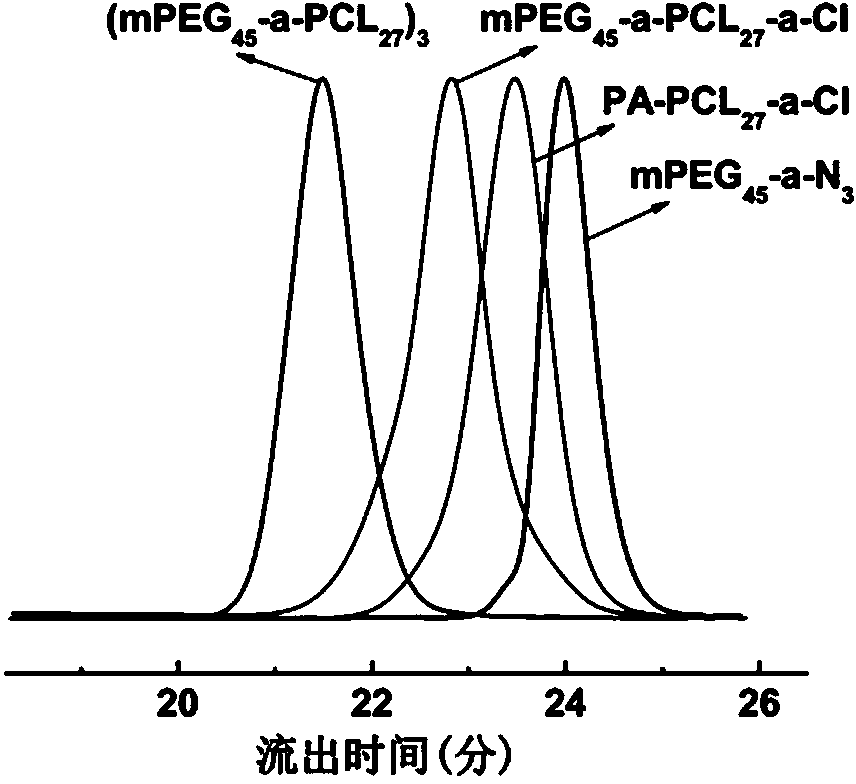
[0084] Example 2: Acid-sensitive polycaprolactone intermediate (PA-PCL) terminated by a monoalkyne group 27 - a -Cl) preparation
[0085] After the branch tube bottle and the ground glass stopper equipped with a stirring bar were processed according to the method of Example 1, they were filled with argon, and under the condition of argon flow, quickly weighed stannous octoate [Sn(Oct) 2 , 0.4050 g, 1 mmol] into the branch tube, pumped and inflated three times, and finally filled with argon, and added anhydrous toluene (20 mL) and ? - caprolactone ( ? -CL, 4.5656 g, 40 mmol) and propargyl alcohol (0.1121 g, 2 mmol). The reaction bottle was filled with argon and moved to a 90 °C oil bath, and the reaction was stirred for 4 hours.
[0086] After the reaction, the product was concentrated, precipitated twice in diethyl ether (150 mL), and filtered with suction to obtain a white solid, which was dried in a vacuum oven at room temperature for 36 hours to obtain polycaprolactone ...
Embodiment 3
[0089] Embodiment Three: Azido-terminated polyethylene glycol monomethyl ether (mPEG 45 - a -N 3 ) preparation
[0090] Weigh polyethylene glycol monomethyl ether (mPEG 45 -OH, 4.0800 g, 2 mmol) and pyridinium 4-methylbenzenesulfonate (0.0503 g, 0.2 mmol) were added to a 100 mL branch vial equipped with a magnetic stirrer, and toluene (30 mL) was added, and the water twice, then dissolve the azeotropic mixture in anhydrous dichloromethane (50 mL), and slowly add 2-chloroethyl vinyl ether (1.0 mL, 10 mmol) and anhydrous dichloromethane in an ice-water bath Add the mixed solution of methane (10 mL) to the branched vial. After the dropwise addition, stir the reaction in an ice-water bath for 0.5 hours.
[0091]After the reaction, add 5% sodium carbonate aqueous solution to terminate the reaction, dilute with dichloromethane (40 mL), then add saturated sodium chloride phosphate buffer solution (pH 10.0, 10 mL), oscillate and let stand. Separate the lower organic phase, extrac...
PUM
| Property | Measurement | Unit |
|---|---|---|
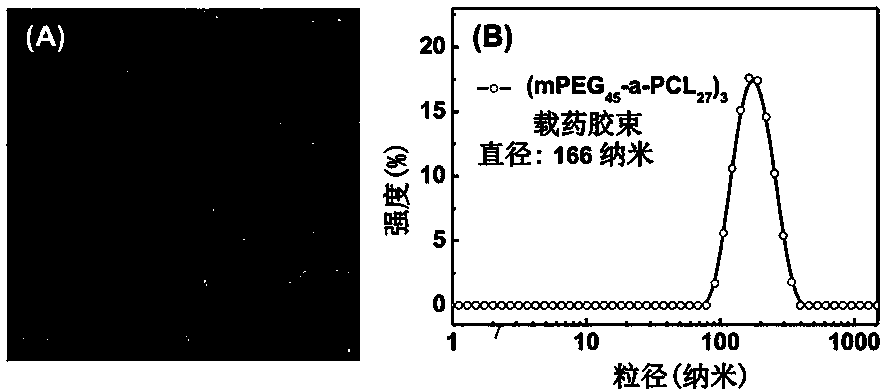
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com