Heterocyclic aromatic micromolecule organic compounds and derivatives, preparation method and medical application
A technology of organic compounds and aromatic heterocycles, applied in the field of aromatic heterocycle small molecule organic compounds and their derivatives, preparation and medical application, can solve the increased risk of cardiovascular and sudden death, no toxic side effects, and short time to market To achieve the effect of improving pharmacokinetic properties, excellent therapeutic effect, and changing physical and chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0189] Example 1: Preparation method of compound
[0190] plan 1
[0191]
[0192] Among them, aromatic thioamide and ethyl bromoacetoacetate or their analogues are heated to 70-100°C for 1-3 hours in a solvent such as ethanol, dioxane, toluene, DMF, and ethylene glycol dimethyl ether. After extraction and purification, it is reduced with borane, lithium aluminum hydride or sodium borohydride to obtain alcohol compounds; the alcohol compounds directly react with halogenated substances to form ethers under the action of potassium tert-butoxide, sodium hydride or potassium carbonate The product, or the alcohol compound after bromination, and alcohol, mercaptan and amine compound to produce ether, thioether and amine compound.
[0193] Scenario 2
[0194]
[0195] Among them, 2-aryl-4-methyl-5-substituent thiazole and N-chlorosuccinimide are refluxed in acetonitrile, tetrahydrofuran, dioxane and toluene to generate chlorinated intermediates, which are combined with alcohols, The merc...
Embodiment 1-1
[0212] Example 1-1, Synthesis of 4-(2-(4-bromobenzyloxy)ethyl)-5-methyl-2-phenylthiazole (LCZ-001)
[0213] Mix thiobenzamide (411mg, 3mmol) and ethyl 4-bromopropionyl acetate (658mg, 4mmol) and dissolve in anhydrous solvent such as ethanol, DMF or ethylene glycol dimethyl ether (25ml), and heat the mixture To 70-100°C. After the completion of the reaction detected by TLC, the obtained mixture was evaporated to dryness of solvent and then extracted twice. The obtained organic phase was washed with saturated brine, evaporated to dryness under reduced pressure, and purified by silica gel column chromatography to obtain the compound ethyl 2-(2- Phenyl-4-thiazolyl-5-methyl)acetate. This compound (741 mg, 3 mmol) was dissolved in 10 ml of ether or dioxane, lithium aluminum hydride (168 mg, 6 mmol) was added under ice bath conditions, the ice bath was removed after stirring for 30 minutes, and the mixture was stirred at room temperature until the reaction was complete. The resulting ...
Embodiment 2
[0214] Example 2: Preparation of compounds LCZ-001 to LCZ-097 (see the reference below for the specific process)
[0215]
[0216]
[0217]
[0218]
[0219]
[0220]
[0221]
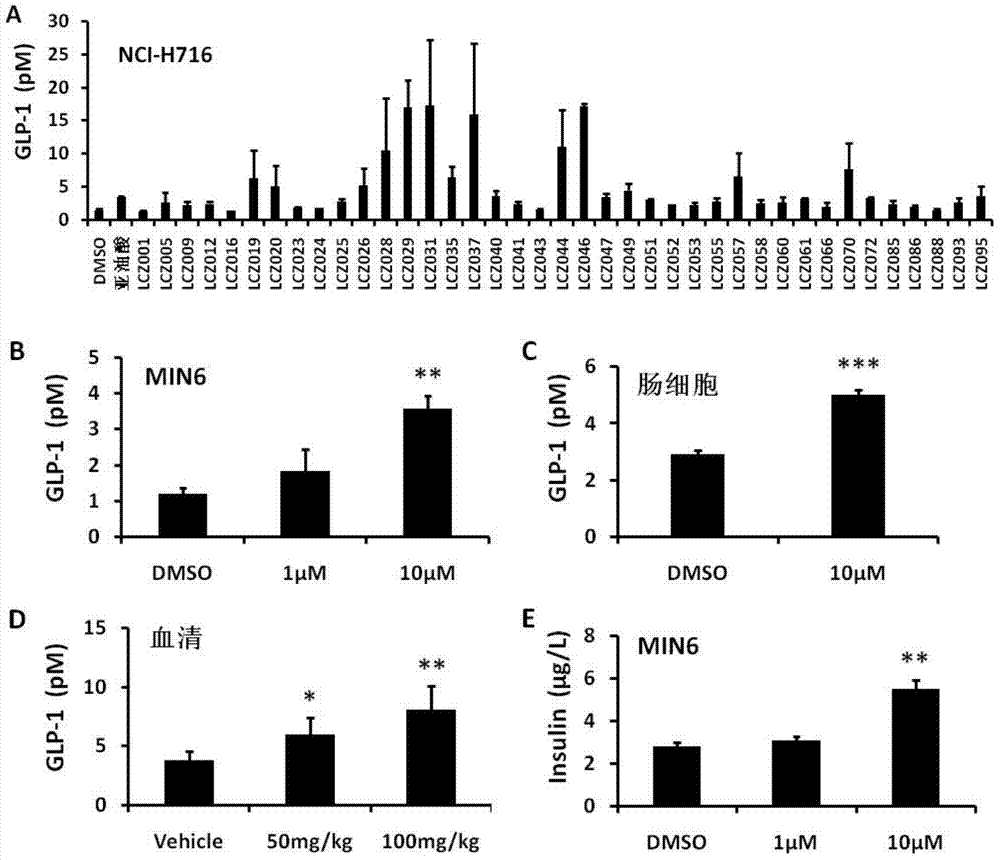
[0222] Example 2: The compound of the present invention can significantly promote the secretion of GLP-1
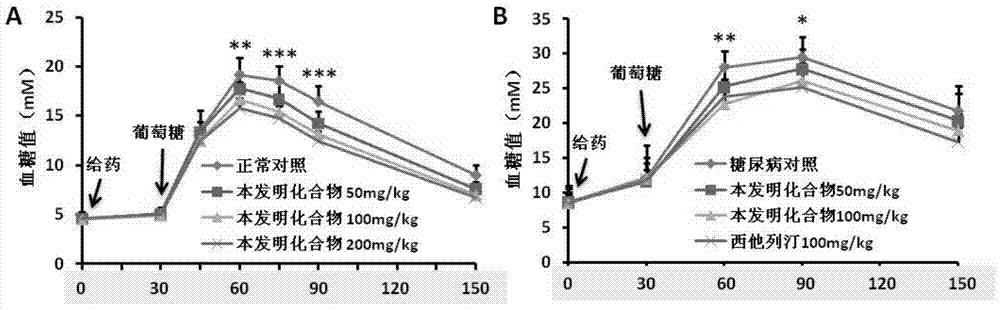
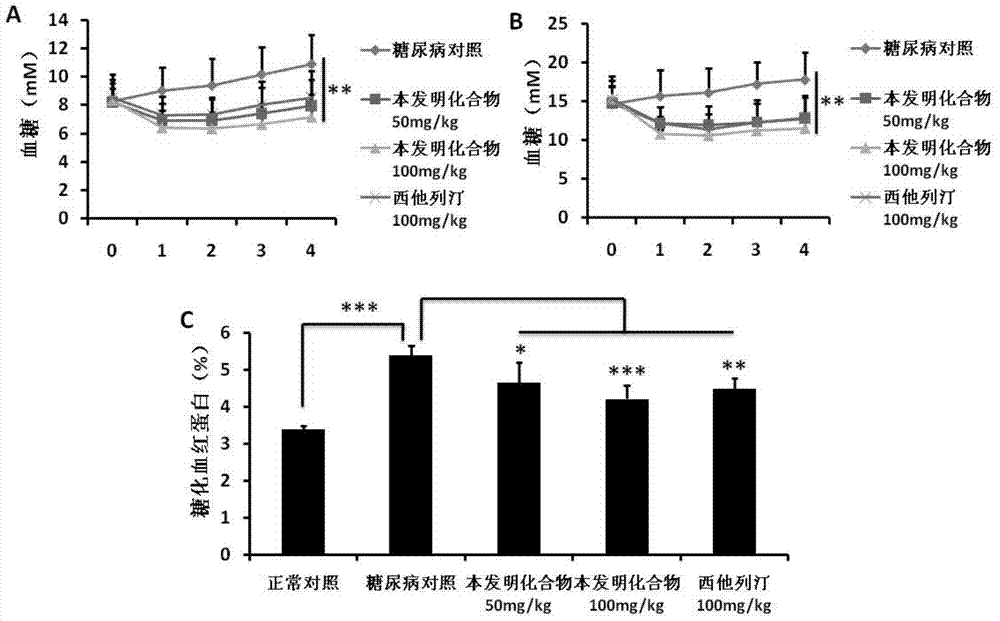
[0223] In order to study the effect of the compound of the present invention on GLP-1 secretion, NCI-H716 was used for drug screening. The result is figure 1 Shown in A. The results show that most of the compounds of the present invention can strongly stimulate NCI-H716 cells to secrete GLP-1, and this effect of promoting GLP-1 secretion has been similarly verified in MIN6, intestinal cells and in vivo experiments in mice ( figure 1 B-D). In addition, the compounds of the present invention can also promote insulin secretion in MIN6. The experimental results indicate that the compound of the present invention may have anti-diabetic effects.
[0224] As mentioned earlier, GLP-1 (glucagon-like peptide 1)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com