Method for preparing lithium iron pyrophosphate used as positive material for lithium ion battery
A technology for lithium iron pyrophosphate and lithium-ion batteries, which is applied in battery electrodes, chemical instruments and methods, circuits, etc. It can solve the problems of difficult to control the morphology of reaction products, difficult to increase discharge specific capacity, and uneven particle size distribution of materials. To achieve the effect of easy control of the operation process, excellent cycle life and good dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] This embodiment includes the following steps:
[0024] Dissolve 0.025mol lithium carbonate, 0.025mol iron nitrate, 0.05mol ammonium dihydrogen phosphate and 0.1mol oxalic acid in 500mL water, control the pH to 5, stir at room temperature for 0.5h to obtain a sol, then raise the temperature to 80°C and keep this temperature for 2h , to form a gel, then dry the gel in a vacuum oven at 120°C, ball mill the obtained xerogel for 1 hour, grind it evenly, and then sinter it at 600°C under the protection of argon for 6 hours, Naturally cooled to room temperature, the finished product lithium iron pyrophosphate (Li 2 FeP 2 o 7 ).
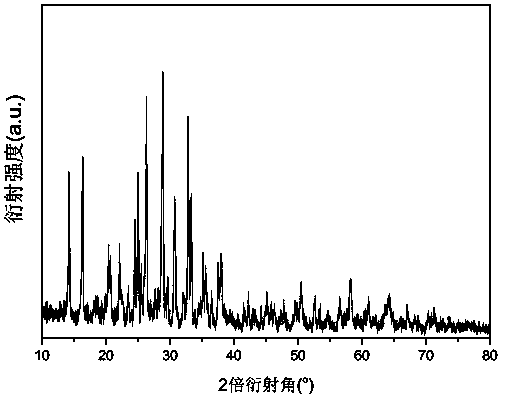
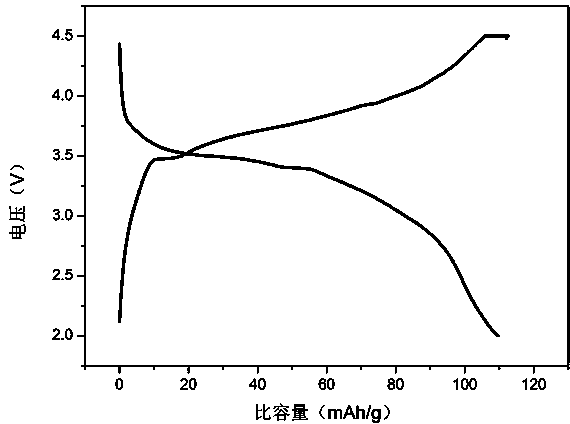
[0025] X-ray powder diffraction analysis shows that the product obtained in this example is pure Li 2 FeP 2 o 7 , no other impurity phases were detected, and the crystallinity was high; from scanning electron microscope analysis, it was known that the particle dispersion of the obtained product was good, and the particle size was 400-600nm;
[...
Embodiment 2
[0028] This embodiment includes the following steps:
[0029] Dissolve 0.05mol lithium hydroxide, 0.025mol ferrous oxalate, 0.025mol pyrophosphoric acid and 0.05mol tartaric acid in 500mL water, control the pH to 4, stir at room temperature for 2h to obtain a sol, then raise the temperature to 60°C and keep it for 10h to make it Form a gel, then dry the gel in a vacuum drying oven at 120°C, ball mill the obtained xerogel for 2 hours, grind it evenly, and then sinter it at 600°C for 20 hours under the protection of nitrogen, and cool it down to room temperature naturally. The finished product lithium iron pyrophosphate (Li 2 FeP 2 o 7 ).
[0030] X-ray powder diffraction analysis shows that the product obtained in this example is pure Li 2 FeP 2 o 7 , no other impurity phases were detected, and the crystallinity was high; from scanning electron microscope analysis, it was known that the particle dispersion of the obtained product was good, and the particle size was 400-50...
Embodiment 3
[0033] This embodiment includes the following steps:
[0034](1) Dissolve 0.05mol lithium acetate, 0.025mol ferrous oxalate, 0.025mol pyrophosphoric acid and 0.05mol tartaric acid in 1000mL water, control the pH to 6, stir at room temperature for 1h to obtain a sol, then raise the temperature to 80°C and keep it for 8h, Make it form a gel, then dry the gel in a vacuum oven at 110°C, ball mill the obtained dry gel for 2 hours, grind it evenly, and then dry it at 500°C under the protection of a mixed gas of hydrogen and argon. Sintering for 10 hours, and naturally cooling to room temperature, the finished product lithium iron pyrophosphate (Li 2 FeP 2 o 7 ).
[0035] X-ray powder diffraction analysis shows that the product obtained in this example is pure Li 2 FeP 2 o 7 , no other impurity phases were detected, and the crystallinity was high; from scanning electron microscope analysis, it was known that the particle dispersion of the obtained product was good, and the part...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Reversible capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com