Method for synthesizing lithium ion battery cathode material lithium iron phosphate through in situ polymerizing and cladding
A technology for lithium ion batteries and cathode materials, which is applied in the field of in-situ polymerization and coating to synthesize lithium iron phosphate cathode materials for lithium ion batteries, can solve problems such as poor cycle performance of materials, achieve good cycle performance, improve lithium ion diffusion rate, The effect of improving electronic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
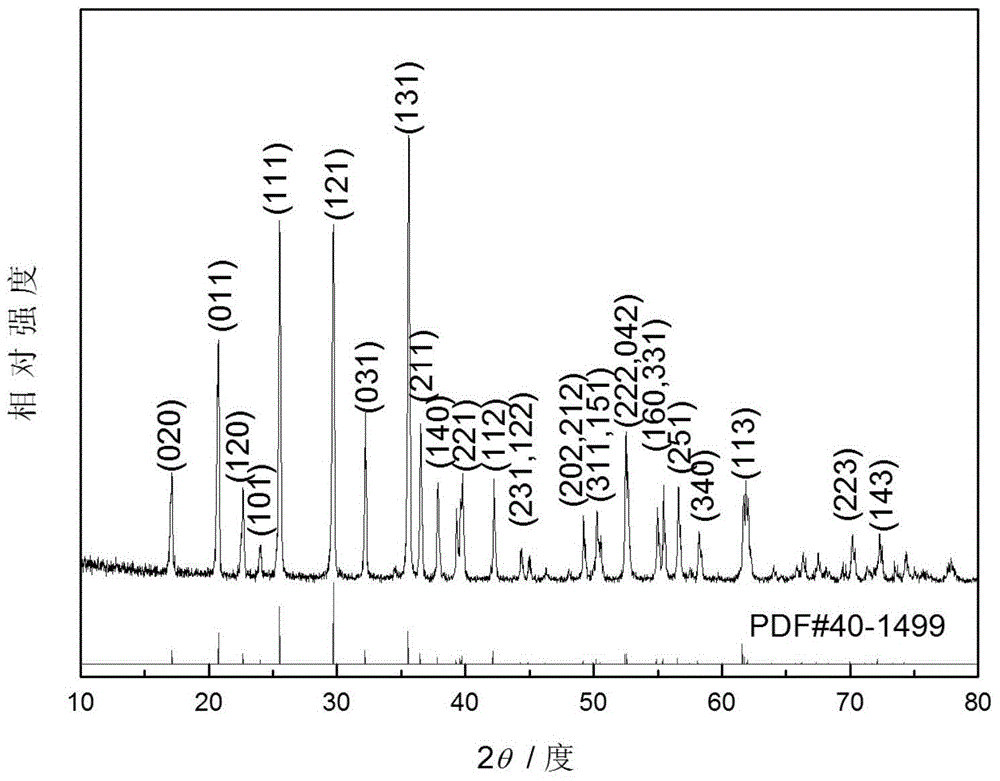
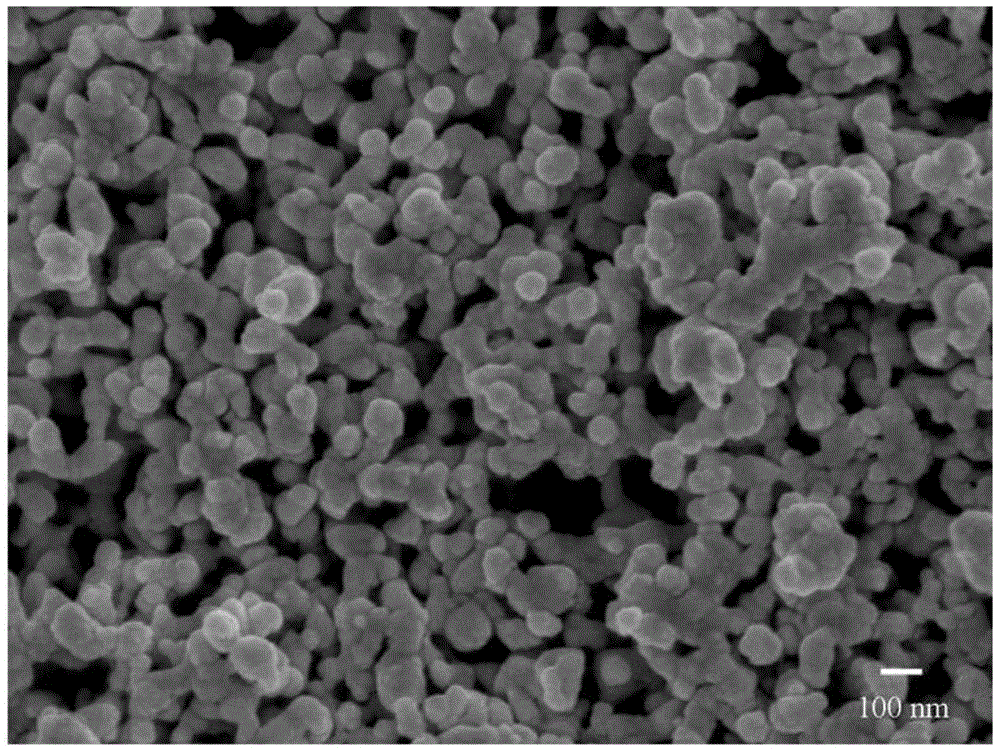
[0035] Add the aniline monomer to 0.3 M NH with vigorous stirring 4 h 2 PO 4 solution, followed by 0.3M FeCl 3 Solution can be added to the above solution, and after reacting for 5 hours, FePO 4 / PANI precursor, then the Li 2 CO 3 The powder is directly added to the above precursor, stirred vigorously on a magnetic stirrer for 2 hours, and then spray-dried to obtain the precursor powder. The precursor powder is heated to 650°C at a heating rate of 15°C / min under an inert atmosphere (nitrogen). And keep the temperature for 5h, then cool to room temperature, and make nano-porous LiFePO 4 / C sample powder. where NH 4 h 2 PO 4 , FeCl 3 , the addition of aniline and lithium carbonate meet: Li:Fe:P=1:1:1 (molar ratio), carbon content is synthetic LiFePO 4 5% (mass ratio) of / C.
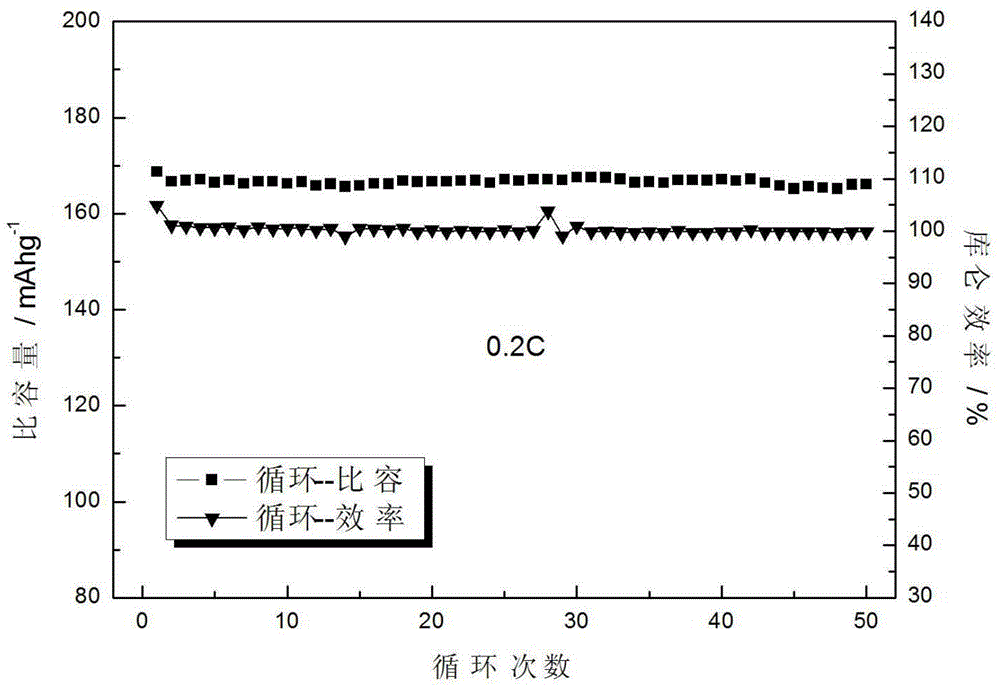
[0036] Positive electrode sheet preparation process: LiFePO 4 / C active material, acetylene black, and binder (polyvinylidene fluoride), mix evenly according to the mass ratio of 90:5:5, make a...
Embodiment 2
[0039] Pyrrole monomer was added to 0.3M ammonium monohydrogen phosphate solution under vigorous stirring, followed by 0.3M FeCl 3 The solution was added to the above solution, and after 7 hours of reaction, black FePO 4 / PPy precursor, then add lithium acetate powder directly to the above precursor, stir vigorously on a magnetic stirrer for 1 h, and then spray dry the precursor powder, and dry the precursor powder in an inert atmosphere (nitrogen) at 30 °C / The heating rate of min is heated to 700°C, and the temperature is maintained for 5 hours, then cooled to room temperature, and nano-sized porous LiFePO is prepared after sieving. 4 / C sample powder. Wherein Li:Fe:P=1:1:1 (molar ratio), the carbon content is synthetic LiFePO 4 / 7% of C (mass ratio).
example 3
[0041] Add acrylonitrile monomer to 0.3M phosphoric acid solution under vigorous stirring, then add 0.3M ferric nitrate solution to the above solution, after 5 hours of reaction, FePO 4 / PAN precursor, then add lithium hydroxide powder directly to the above precursor, stir vigorously on a magnetic stirrer for 2h, and then spray dry the precursor powder, and dry the precursor powder in an inert atmosphere (nitrogen) at 1 °C / min heating rate to 500 ° C, and keep the temperature for 15 hours, then cooled to room temperature, after passing through the sieve to produce nano-porous LiFePO 4 / C sample powder. Wherein Li:Fe:P=1:1:1 (molar ratio), the carbon content is synthetic LiFePO 4 10% of / C (mass ratio).
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com