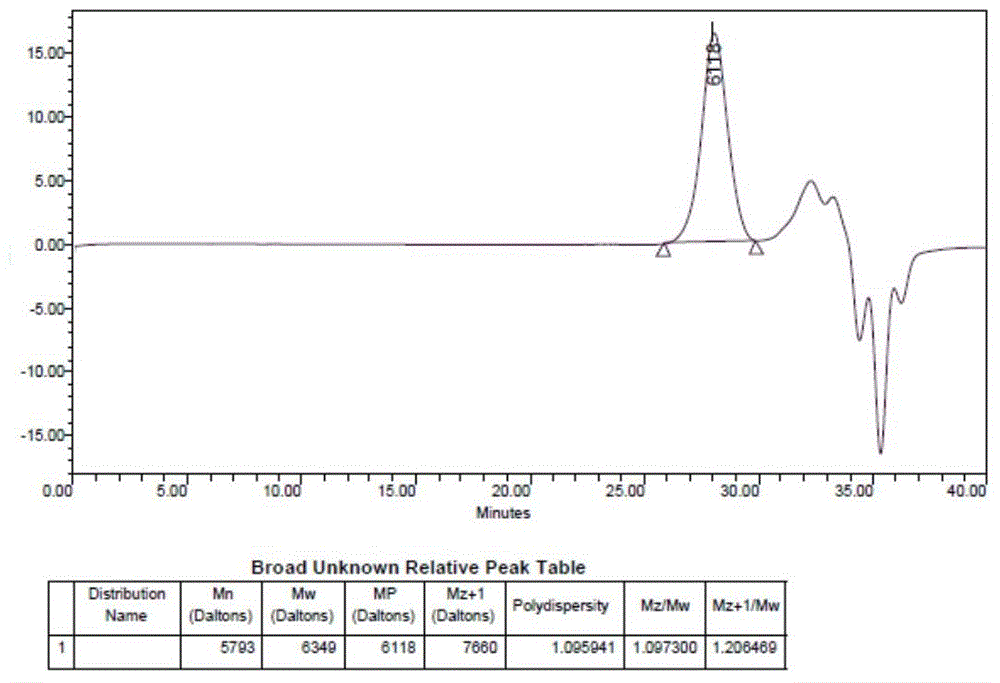
Paclitaxel micelle drug load system and preparation method thereof
A drug-carrying system, paclitaxel technology, applied in the direction of pharmaceutical formulations, anti-tumor drugs, drug combinations, etc., can solve the problems of slow dilution of injections, inconsistent drug levels, limited capacity, etc., to achieve good industrial application prospects and convenient storage and transport, increase the effect of force
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The synthesis of embodiment 1mPEG-PLA-Phe (Boc)
[0037] 5g of methoxypolyethylene glycol with a molecular weight of 2000, 6g of lactide, 6mg of stannous octoate, and 10ml of toluene were added to the polymerization bottle, and the toluene was removed by vacuum at 50°C to remove the moisture in the system, and the vacuum-sealed polymerization bottle was placed at 100 o Polymerize at ℃ for 24 hours, dissolve the product with dichloromethane, and precipitate with ether to obtain methoxypolyethylene glycol-polylactide block copolymer (MPEG-PLA).
[0038] Dissolve 6.65g of Boc phenylalanine in 50ml of anhydrous ethyl acetate, add 3.5ml of triethylamine, add 3.05ml of pivaloyl chloride after the solution is cooled to -10°C, heat up the reactant to 0°C for 2 hours, then continue the reaction at room temperature 2h. The insoluble matter was removed by filtration, and the ethyl acetate was removed by rotary evaporation to obtain a white solid which was Boc phenylalanine-pivali...
Embodiment 2
[0041] Preparation of embodiment 2MPEG-PLA-Phe(Boc) / paclitaxel micelles
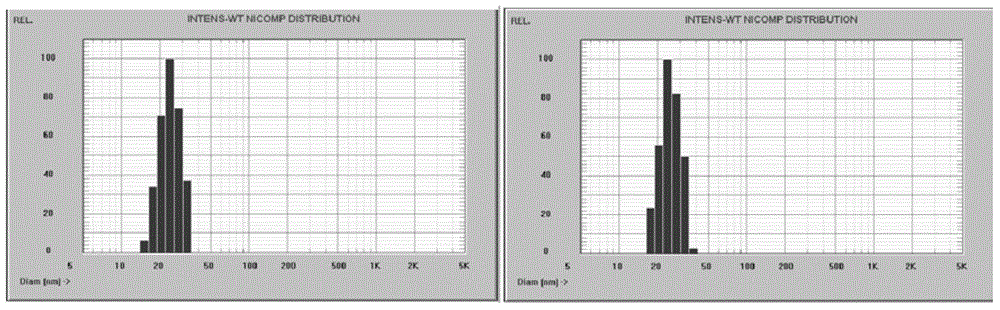
[0042] Dissolve 20mg paclitaxel, 100mg MPEG-PLA-Phe (Boc) in 5ml ethanol, remove the solvent by rotary evaporation at 55°C, add 5ml ultrapure water to dissolve the drug film, and the obtained micellar solution is filtered through a 0.22μm sterile membrane and freeze-dried to obtain paclitaxel gel Bunch of freeze-dried powder. Its resolubility is good, and the change of particle size distribution before and after reconstitution is small, and the detailed results are shown in the attached figure 2 And attached image 3 .
[0043] Test example 3 pharmacokinetic test
[0044] A. Experimental animals:
[0045] Male SD rats, weighing 240±20g, were randomly divided into four groups, with 6 rats in each group, and they were reserved.
[0046] B. Experimental preparation:
[0047] Preparation I: It is paclitaxel micelle freeze-dried powder preparation for testing, prepared according to Example 2, batch num...
Embodiment 4
[0057] Embodiment 4 pharmacodynamics test
[0058] 4.1 Inhibitory effect of paclitaxel micelles for injection on xenograft tumors of prostate cancer PC-3A doxorubicin-resistant cells in nude mice
[0059] Male BALB / c nude mice were subcutaneously inoculated with 5×10 6 PC-3A cells. After about a week, the average volume of tumors in tumor-bearing mice reached 100mm 3 For the above, 30 tumor-bearing mice were randomly stratified and grouped according to tumor volume, respectively: vehicle group, preparation I (20 mg / kg, derived from Example 2), preparation II, preparation III, and preparation IV refer to patent CN201110231519.7 Prepared with CN01809632.8 (20mg / kg), administered intravenously, once every 3 days, for a total of 3 times. During the experiment, the animal tumor volume was determined every week (calculation formula ab 2 / 2, a and b are the length and width of the tumor, respectively) and body weight. The result is as Figure 5 As shown, in the late stage of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com