A comprehensive utilization method of silicate minerals
A silicate and mineral technology, applied in the field of comprehensive utilization of non-metallic ore and hydrometallurgy, can solve the problems of not providing, not meeting spray drying, and not being able to prepare high-purity iron oxide red products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
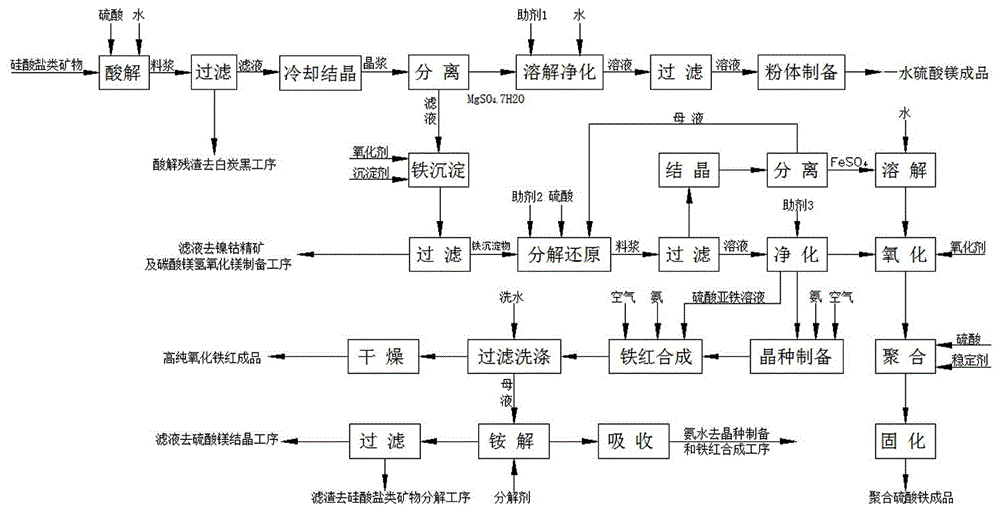
[0109] Such as figure 1As shown, the comprehensive utilization method of the silicate minerals, the processed ore is Sichuan Ya'an asbestos tailings, chemical composition: MgO: 35.74%, Fe 2 o 3 : 6.22%, SiO 2 : 41.81%, NiO: 0.37%, CoO: 0.031%, follow the steps below:
[0110] (1) Pulverize the asbestos tailings to 120 mesh, weigh 100kg of asbestos tailings powder and the secondary acid hydrolysis solution at a solid-to-liquid ratio of 1:2.5 (secondary acid hydrolysis solution 250L) and mix them in the primary acid hydrolysis reactor, After stirring evenly, add 1 kg of activator fluorite, then raise the temperature and add sulfuric acid, control the reaction temperature to 80°C, keep the reaction for 60 minutes, and control the end point pH value to 1. After the reaction was finished, 139kg of the first-level acidolysis residue (50.5% water content, 68.8kg in dry form) and 255L of the first-level acidolysis solution were obtained by filtration. MgSO in primary acid hydrolys...
Embodiment 2
[0125] The raw ore processed is asbestos tailings in Ya'an, Sichuan, chemical composition: SiO 2 : 41.81%, MgO: 35.74%, Fe 2 o 3 : 6.22%, NiO: 0.37%, CoO: 0.031%. Follow the steps below:
[0126] (1) Pulverize the asbestos tailings to 120 mesh, weigh 100kg of asbestos tailings powder and the secondary acid hydrolysis solution at a solid-to-liquid ratio of 1:5 (secondary acid hydrolysis solution 500L) and mix them in the primary acid hydrolysis reactor, After stirring evenly, add 1 kg of activator industrial calcium fluoride, then raise the temperature and add sulfuric acid, control the reaction temperature to 75°C, keep the reaction for 90 minutes, and control the end point pH value to 1. After the reaction was completed, 120 kg of the primary acidolysis residue (49.5% water content, 60.6 kg in dry form) and 500 L of the primary acidolysis solution were obtained by filtration. MgSO in primary acid hydrolysis solution 4 Concentration 213g / l, FeSO 4 The concentration was 2...
Embodiment 3
[0141] The raw ore processed is Serpentine Mine in Inner Mongolia Sunite Left Banner, chemical composition: MgO: 37.81%, SiO 2 : 36%, Fe 2 o 3 : 5.93%, Ni: 0.20%, Co: 0.009%.
[0142] (1) Crush the serpentine to 100 mesh, weigh 100kg of serpentine ore powder and the secondary acid hydrolysis solution at a solid-to-liquid ratio of 1:2.5 (secondary acid hydrolysis solution 250L) and mix them in the primary acid hydrolysis reactor , after stirring evenly, add 1kg of activator, then raise the temperature and add sulfuric acid, control the reaction temperature to 85°C, keep the reaction for 60min, and control the end point pH value to 1.2. After the reaction was completed, 174 kg of the primary acidolysis residue (50.5% water content, 86 kg in dry form) and 262 L of the primary acidolysis solution were obtained by filtration. MgSO in primary acid hydrolysis solution 4 Concentration 432g / l, FeSO 4 The concentration was 42.7 g / l.
[0143] Mix the primary acidolysis residue and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com