Riemerella anatipestifer OmpH intercepted recombinant protein, and preparation method and application thereof
A technology of Riemerella anatipestiferus and recombinant protein, which is applied in the field of bioengineering, can solve the problems of low sensitivity and specificity, no research and application research reports on Riemerella anatipestifer, and achieve stable performance and market application The effect of wide prospects and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of embodiment 1 Riemerella anatipestifer OmpH truncated recombinant protein
[0045] 1. Main experimental materials
[0046] Plasmid T-VectorpMD19 (simple), MaxDNAPolymerase was purchased from Dalian Bao Biological Engineering Co., Ltd.; prokaryotic expression plasmid pET32a (+), Novagen product; clone host bacteria E.coliDH5α, expression host bacteria E.coliBL21 (DE3) and RA-CH-1 strains, from Sichuan Provided by the Poultry Disease Research Center of Agricultural University.
[0047] 2 Experimental methods
[0048] 2.1 Cloning of RA-CH-1OmpH truncated gene
[0049] 2.1.1 Primer design
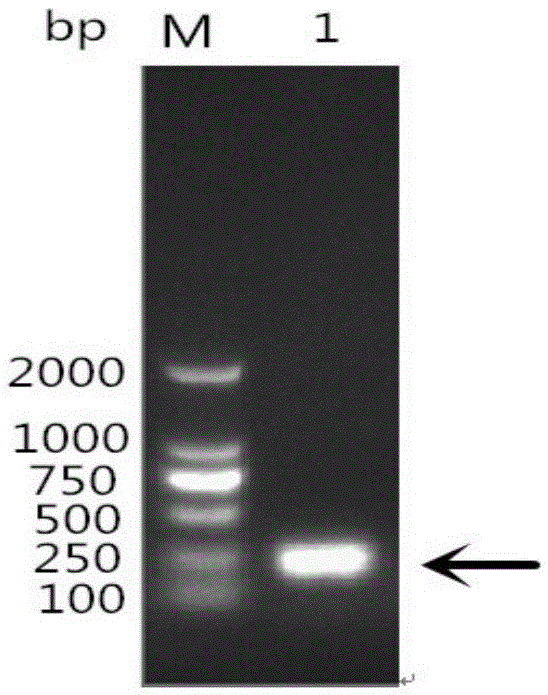
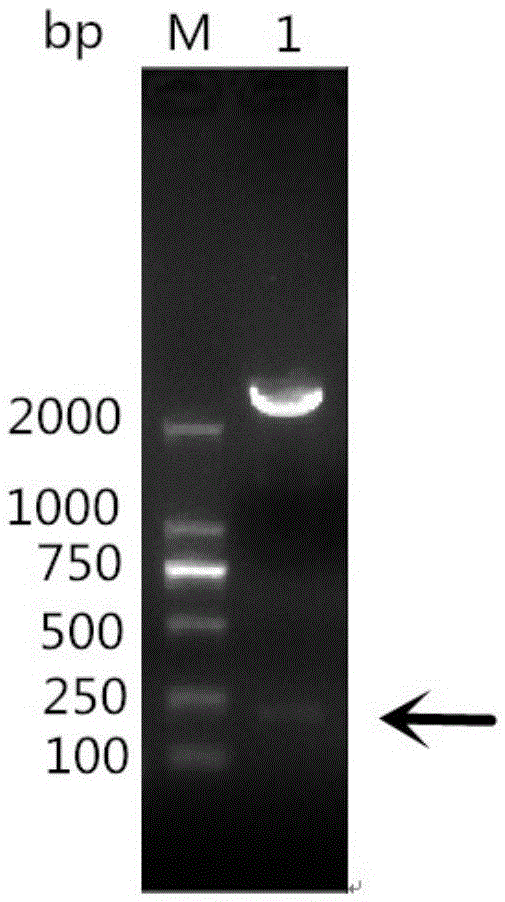
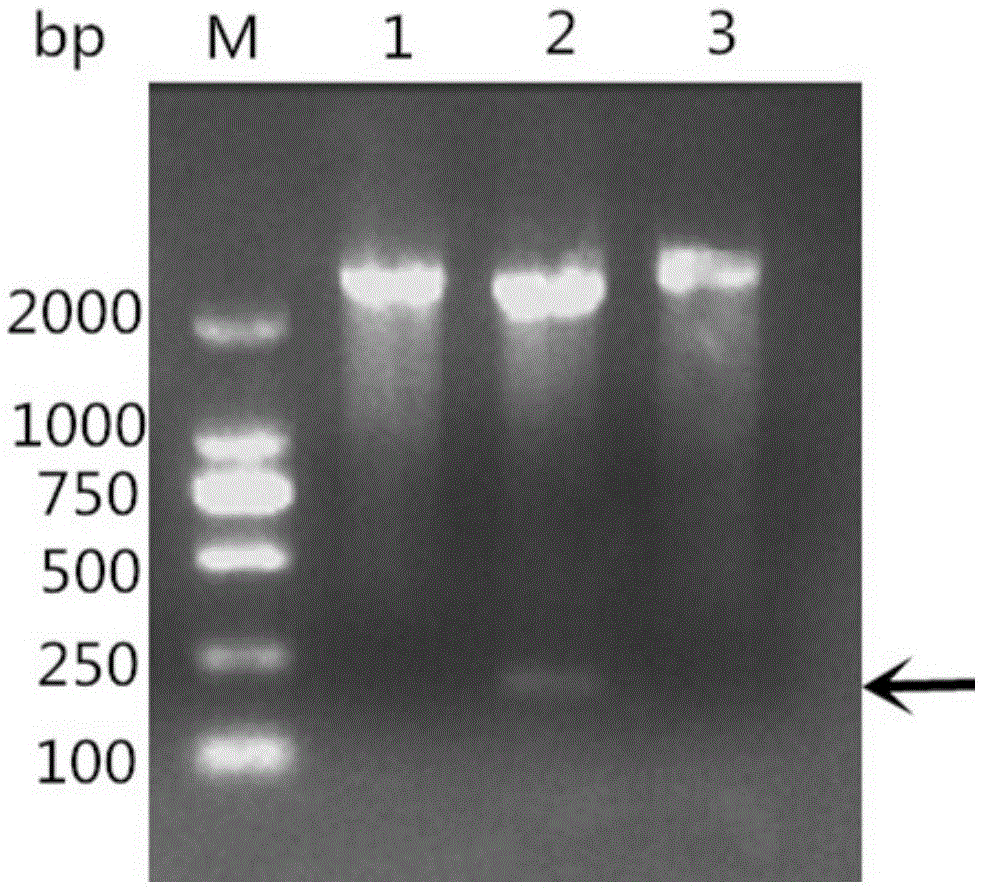
[0050] According to the existing RA-CH-1OmpH gene sequence in GenBank, the main antigenic domain (81aa-151aa and named as OmpHM) was selected, and a pair of primers were designed using PrimerPremier5.0 software. Upstream primer as shown in SEQIDNO:3: 5'-CGC GGATCC GAGGCTCAGAGAACTGCT-3' (the underlined part is the BamHI site); the downstream primer shown in SEQ I...
Embodiment 2
[0131] Embodiment 2 Purification of Riemerella anatipestifer OmpH truncated recombinant protein
[0132] After the expression product containing the OmpH truncated recombinant protein of Riemerella anatipestifer prepared in Example 1 was collected from the bacteria, ultrasonically disrupted, and supernatant collected, the recombinant protein was purified by nickel agarose gel affinity chromatography. OmpH truncated recombinant protein. The UV curve of the protein sample after column purification showed three peaks, peak 1 was the breakthrough peak, peak 2 was the elution peak of 50 mmol imidazole, and peak 3 was the elution peak of 100 mmol imidazole. Collect different concentrations of imidazole elution peaks simultaneously, carry out SDS-PAGE electrophoresis, check purity and concentration, the result shows: only contain a large amount of high-purity OmpH truncated recombinant proteins in the 100mmol imidazole elution peak ( Figure 8 ). After the purified OmpH truncated r...
Embodiment 3
[0133] Western-blot (western blot) of embodiment 3 Riemerella anatipestifer OmpH truncated recombinant protein
[0134] 1. Experimental method
[0135] The recombinant protein containing the OmpH truncated fragment of Riemerella anatipestifer obtained in Example 1 was used as a probe for detecting the serum antibody of Riemerella anatipestifer.
[0136] 1SDS-PAGE gel preparation
[0137] 1. Preparation of 112% separating gel
[0138] 1215 μl of deionized water, 950 μl of 1.5MTris-HCl (PH=8.8), 37.5 μl of 10% SDS, 37.5 μl of 10% AP, 1.5 μl of TEMED, and 1.5 ml of 30% acrylamide.
[0139] Preparation of 1.25% Stacking Gel
[0140] 700 μl of deionized water, 125 μl of 1.0MTris-HCl (pH=6.8), 10.0 μl of 10% SDS, 10.0 μl of 10% AP, 1.0 μl of TEMED, and 165 μl of 30% acrylamide.
[0141] 2 Processing of protein samples
[0142] Add an appropriate amount of SDS-PAGE protein loading buffer (0.5MTris-HCl (PH=6.81.2ml), glycerol 1ml, deionized water 4.8ml, 10% SDS2.0ml, 0.1% BPB in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com