Method for reducing carbon dioxide through inorganic semiconductor photocatalysis system
An inorganic semiconductor and carbon dioxide technology, which is applied in the preparation of carbon dioxide reduction method, chemical instruments and methods, preparation of organic compounds, etc., can solve problems such as excessively wide band gap, and achieve the effects of simple composition, high utilization rate of visible light, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: the aqueous phase preparation of CdSe quantum dot
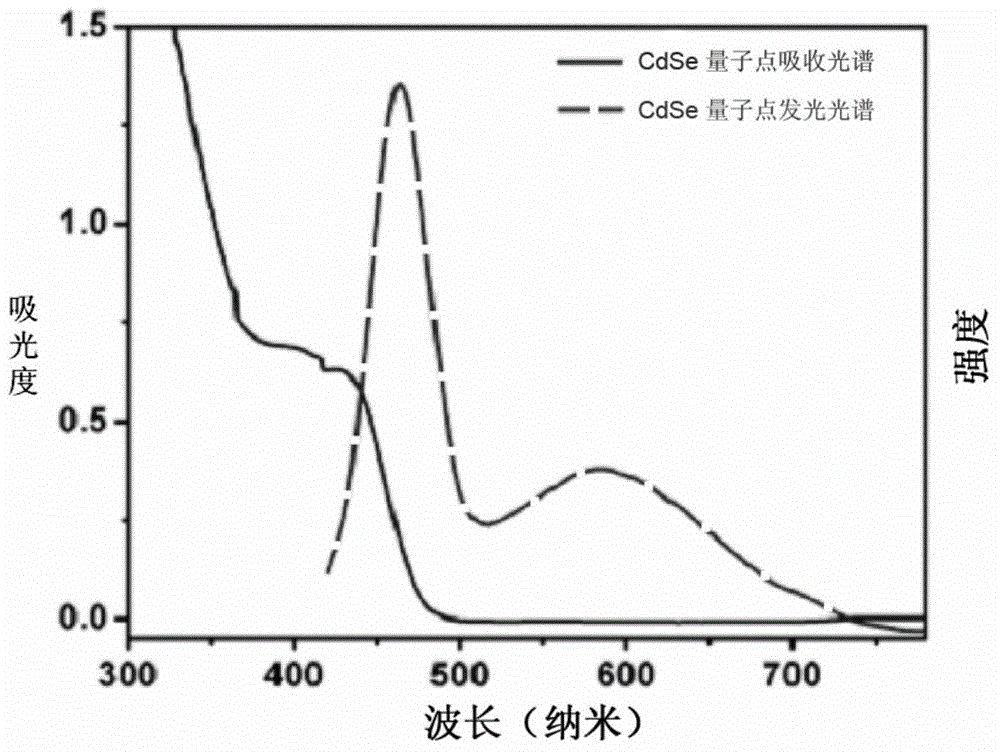
[0064] The experimental steps include: ① preparation of Na 2 SeSO 3 : First weigh 40.0mg selenium powder (0.5mmol) and add to 100mL Na 2 SO 3 (189 mg) in aqueous solution, degassed for 30 minutes. Heat to reflux until the selenium powder is completely dissolved to obtain clear and transparent Na 2 SeSO 3 Solution, stored away from light under an inert atmosphere; ②Synthesis of water-soluble CdSe quantum dots: add 46mg CdCl to a 500mL single-necked round bottom flask 2 ·5 / 2H 2 O (0.2mmol), 190mL deionized water and 26μL mercaptopropionic acid (0.3mmol), adjust the pH value to 11.00 with 1.0mol / L NaOH, and pass argon for 30 minutes; then use a syringe to take 10mL of fresh Na 2 SeSO 3 The solution was quickly injected into the reaction solution of the single-necked flask, continued degassing for 20 minutes, and refluxed for 2.0-4.0h to obtain a yellow-green solution, and the synthesized CdSe quantum...
Embodiment 2
[0065] Embodiment 2: CdSe nanorod oil phase synthesis
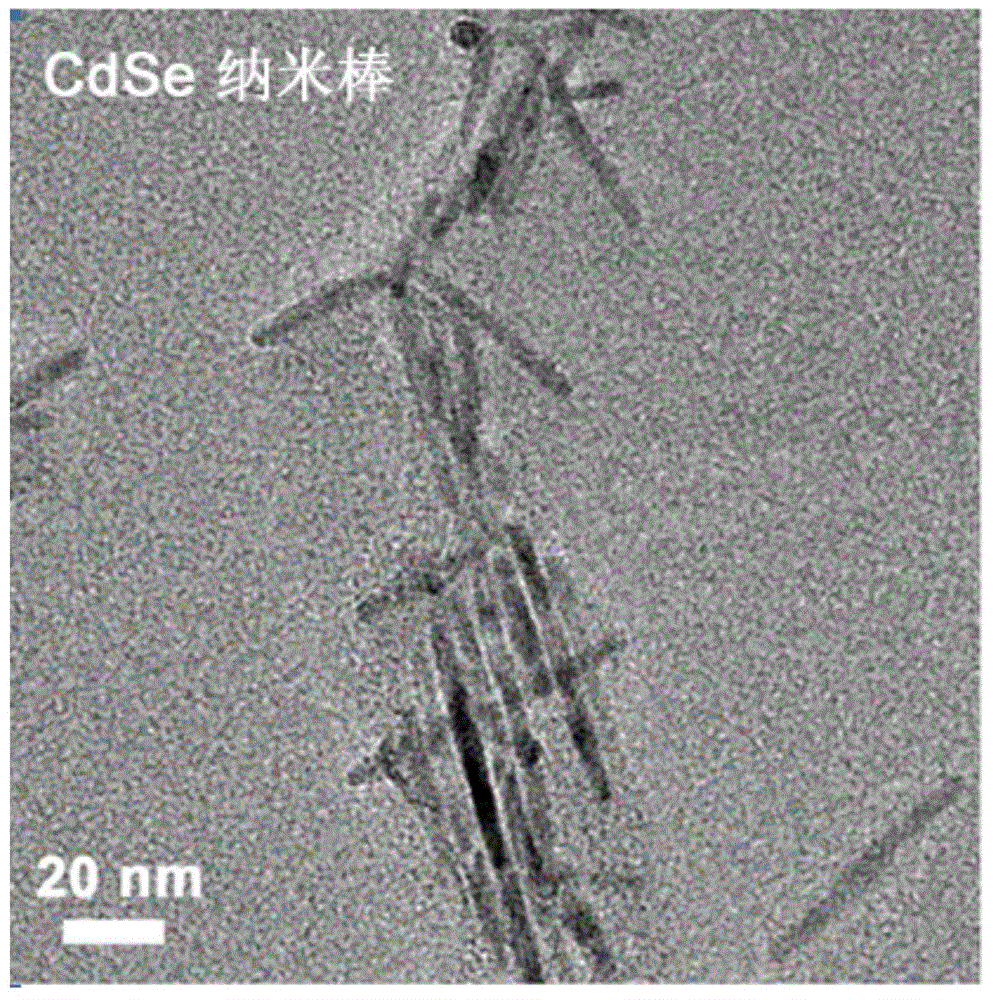
[0066] Add 0.06g CdO, 0.28g octylated diphenylamine (abbreviation: ODPA) and 3g tri-n-octylphosphine oxide (abbreviation: TOPO) into a 25mL three-necked flask, heat to 300°C under argon atmosphere, and dissolve CdO into the solution Clear and transparent, inject 1.5g trioctylphosphine (abbreviation: TOP), the temperature rises to 350 ° C, inject the precursor of Se (0.058g Se+0.36g TOP), where [Cd]:[Se] (molar ratio) = 2:3, the reaction was stopped after 5s to obtain CdSe seeds with a diameter of about 2.6nm. CdSe nanorods can be obtained by changing the ratio of precursor, stabilizer and temperature, figure 2 Electron micrographs of CdSe nanorods synthesized in oil phase.
Embodiment 3
[0067] Embodiment 3: the aqueous phase synthesis of CdTe quantum dot
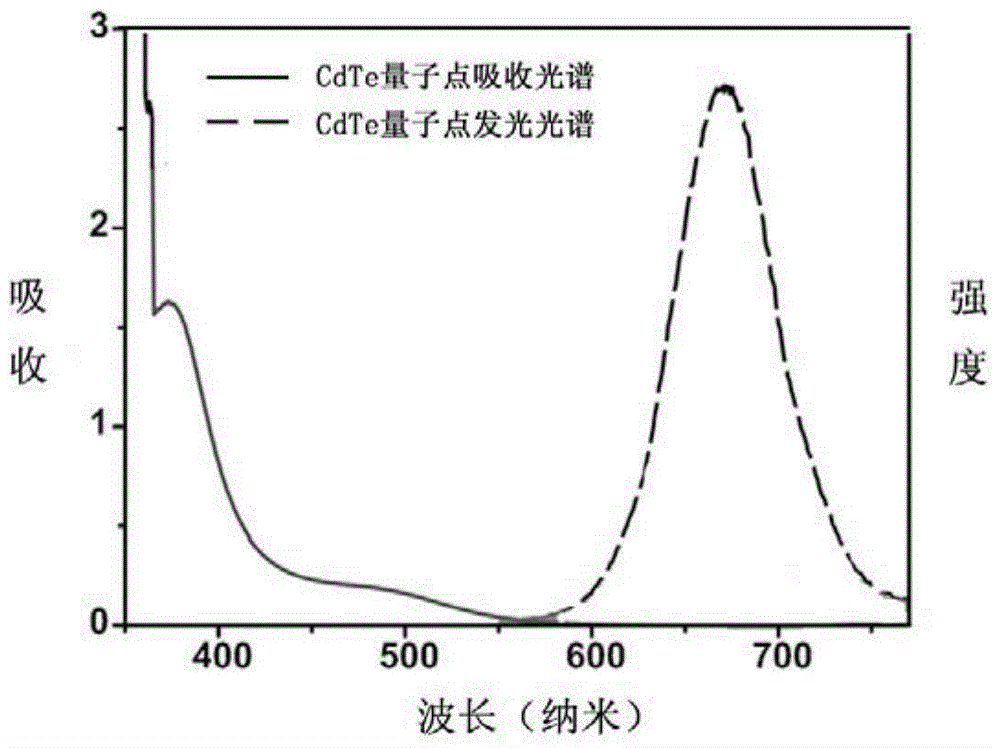
[0068] ① Synthesis of NaHTe: Put a small magnet in a 3.0mL glass bottle, adjust the rotation speed so that it just rotates. Prepare an ice bath, weigh 100mg NaBH 4 Add to the glass bottle, add 1.0 mL of pre-cooled distilled water, seal it with a parafilm, make a small hole, stir until clear, add 127 mg of Te powder, ice bath, and stir for 4.0 h. ②Synthesis of CdTe quantum dots: add 46mg CdCl to a 250mL round bottom flask 2 ·5 / 2H 2 O, 200mL deionized water and 26μL mercaptopropionic acid, stirred; then adjust the pH value to 11.2 with 1.0M sodium hydroxide, and flow argon for 30min. Take 0.5mL freshly prepared NaHTe and add it into the reaction system, continue degassing for 20min, and then reflux for 2.0h to obtain CdTe quantum dots. image 3 Spectral characterization of the synthesized CdTe quantum dots.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com