Method for preparing hydrogen fluoride and sodium fluoride by use of fluosilicic acid
A technology of fluorosilicic acid and sodium fluoride, applied in the direction of fluorine/hydrogen fluoride, hydrogen fluoride, alkali metal fluoride, etc., can solve the problems of high impurity content of by-products, increase production cost, and small usable value, and achieve high product purity. , the effect of saving raw materials, good economic benefits and environmental benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
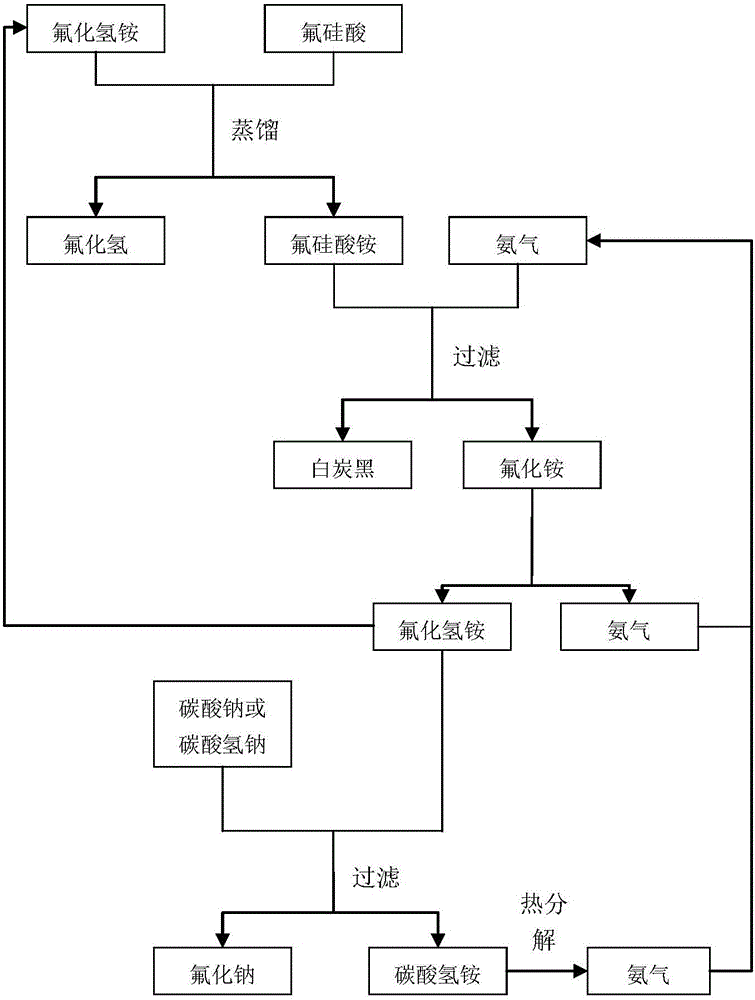
[0038] Preparation of hydrogen fluoride and sodium fluoride with fluorosilicic acid, the process flow chart is as follows figure 1 shown.
[0039] 1. For the first production cycle, follow the steps below:
[0040]1) Take 50 kg of fluorosilicic acid solution with a mass concentration of 20% and 8.0 kg of ammonium bifluoride, place them in a tetrafluoroethylene-lined still, set the temperature at 95° C., and distill for 5 hours. The fractions are condensed to obtain hydrofluoric acid solution, containing Hydrogen fluoride 5.5kg, the residue at the bottom of the kettle is ammonium fluorosilicate solid.
[0041] 2) Add 55 kg of ammonia water with a mass fraction of 25% to the bottom of the kettle, set the temperature at 30° C., and react for 120 minutes. Filter the reaction system to obtain white carbon black and a mixed solution containing ammonium fluoride and ammonia water. The mixed solution was heated for 90 minutes at a temperature of 220° C., ammonia gas was collected to...
Embodiment 2
[0052] 1. The first production cycle:
[0053] 1) Take 50kg of fluorosilicic acid solution with a mass concentration of 25% and 14.8kg of ammonium bifluoride, place it in a still lined with tetrafluoroethylene, set the temperature at 140°C, and distill for 2.5h. The fractions are condensed to obtain hydrofluoric acid containing Hydrogen fluoride 6.9kg, the residue at the bottom of the kettle is ammonium fluorosilicate solid.
[0054] 2) Add 30 kg of ammonia water with a mass fraction of 25% to the bottom of the kettle, set the temperature at 65° C., and react for 60 minutes. Filter the reaction system to obtain white carbon black and a mixed solution containing ammonium fluoride and ammonia water. The mixed solution was heated for 240 minutes at a temperature of 110° C., ammonia gas was collected to obtain ammonia water, and the solid ammonium bifluoride product was weighed to obtain a mass of 19.6 kg.
[0055] 3) Take 4.8 kg of ammonium bifluoride obtained in step 2) and dis...
Embodiment 3
[0063] 1. The first production cycle:
[0064] 1) Take 50 kg of fluorosilicic acid solution with a mass concentration of 15% and 5.4 kg of ammonium bifluoride, place them in a tetrafluoroethylene-lined distillation kettle, set the temperature at 150°C, and distill for 1 hour. The distillate is condensed to obtain hydrofluoric acid containing 3.8% hydrogen fluoride kg, the residue at the bottom of the kettle is ammonium fluorosilicate solid.
[0065] 2) Add 11.0 kg of ammonia water with a mass fraction of 25% to the bottom of the kettle, set the temperature at 70° C., and react for 60 minutes. Filter the reaction system to obtain a mixed solution containing white carbon black and ammonium fluoride and ammonia water. The mixed solution was heated for 150 minutes at a temperature of 180° C., ammonia gas was collected to obtain ammonia water, and the solid ammonium bifluoride product was weighed to obtain a mass of 8.1 kg.
[0066] 3) Take 2.7 kg of ammonium bifluoride obtained i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com