Method for preparing oil-soluble hydroxyapatite nanometer particles based on nonaqueous system
A technology of hydroxyapatite and nanoparticles, which is applied in the direction of nanotechnology, nanotechnology, nanotechnology, etc. for materials and surface science, and can solve the crystallinity, morphology, and uniformity of hydroxyapatite flake nanocrystals Nano size cannot achieve optimization, surface modifiers are difficult to biocompatibility, modifiers cannot be covalently bonded, etc., to achieve good shape controllability, good dispersion, and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1) Dissolve 0.02mol calcium nitrate in 20 grams of ethylene glycol to obtain solution A;
[0036] 2) Dissolve 0.005 mol of potassium hydroxide in 20 grams of ethylene glycol to obtain solution B;
[0037] 3) Add solution A obtained in step 1), 20 grams of propylene glycol, 0.01 mol oleic acid and solution B obtained in step 2) into a three-necked flask for mixing, and stir to obtain solution C;
[0038] The mol ratio of described oleic acid and calcium nitrate is 1 / 2;
[0039] The molar ratio of described oleic acid and potassium hydroxide is 2.
[0040] 4) Dissolve 0.012 mol of disodium hydrogen phosphate in 20 grams of ethylene glycol to obtain solution D;
[0041] 5) Under stirring conditions, slowly drop the solution D obtained in step 4) into the solution C obtained in step 3), and continue stirring for 10 minutes after the dropwise addition to obtain a mixed solution;
[0042] The mol ratio of described calcium nitrate and disodium hydrogen phosphate is 5:3;
...
Embodiment 2
[0045] Embodiment 2, with embodiment 1, difference is,
[0046] 1) Dissolve 0.02mol calcium nitrate in 20 grams of ethylene glycol to obtain solution A;
[0047] 2) Dissolve 0.01mol of sodium hydroxide in 20 grams of ethylene glycol to obtain solution B;
[0048] 3) Add solution A obtained in step 1), 30 grams of propylene glycol, 0.01 mol oleic acid and solution B obtained in step 2) into a three-necked flask for mixing, and stir to obtain solution C;
[0049] The mol ratio of described oleic acid and calcium nitrate is 1 / 2;
[0050] The molar ratio of described oleic acid and sodium hydroxide is 1.
[0051] 4) Dissolve 0.012 mol of dipotassium hydrogen phosphate in 20 grams of ethylene glycol to obtain solution D;
[0052] 6) The mixed solution obtained in step 5) was heated to 120° C. for 12 hours of reaction, and then naturally cooled to room temperature to obtain a reaction solution.
Embodiment 3
[0053] Embodiment 3, with embodiment 1, difference is,
[0054]1) Dissolve 0.02mol calcium nitrate in 20 grams of ethylene glycol to obtain solution A,
[0055] 2) 0.013mol ethylenediamine was dissolved in 20 grams of ethylene glycol to obtain solution B,
[0056] 3) Add solution A obtained in step 1), 40 grams of propylene glycol, 0.018mol oleic acid and solution B obtained in step 2) into a three-necked flask for mixing, and stir to obtain solution C;
[0057] The mol ratio of described oleic acid and calcium nitrate is 9 / 10;
[0058] The molar ratio of oleic acid and ethylenediamine is 18 / 13.
[0059] 4) Dissolve 0.012 mol of trisodium phosphate in 20 grams of ethylene glycol to obtain solution D;
[0060] 6) The mixed solution obtained in step 5) was heated to 150° C. for 3 hours to react, and then naturally cooled to room temperature to obtain a reaction solution.
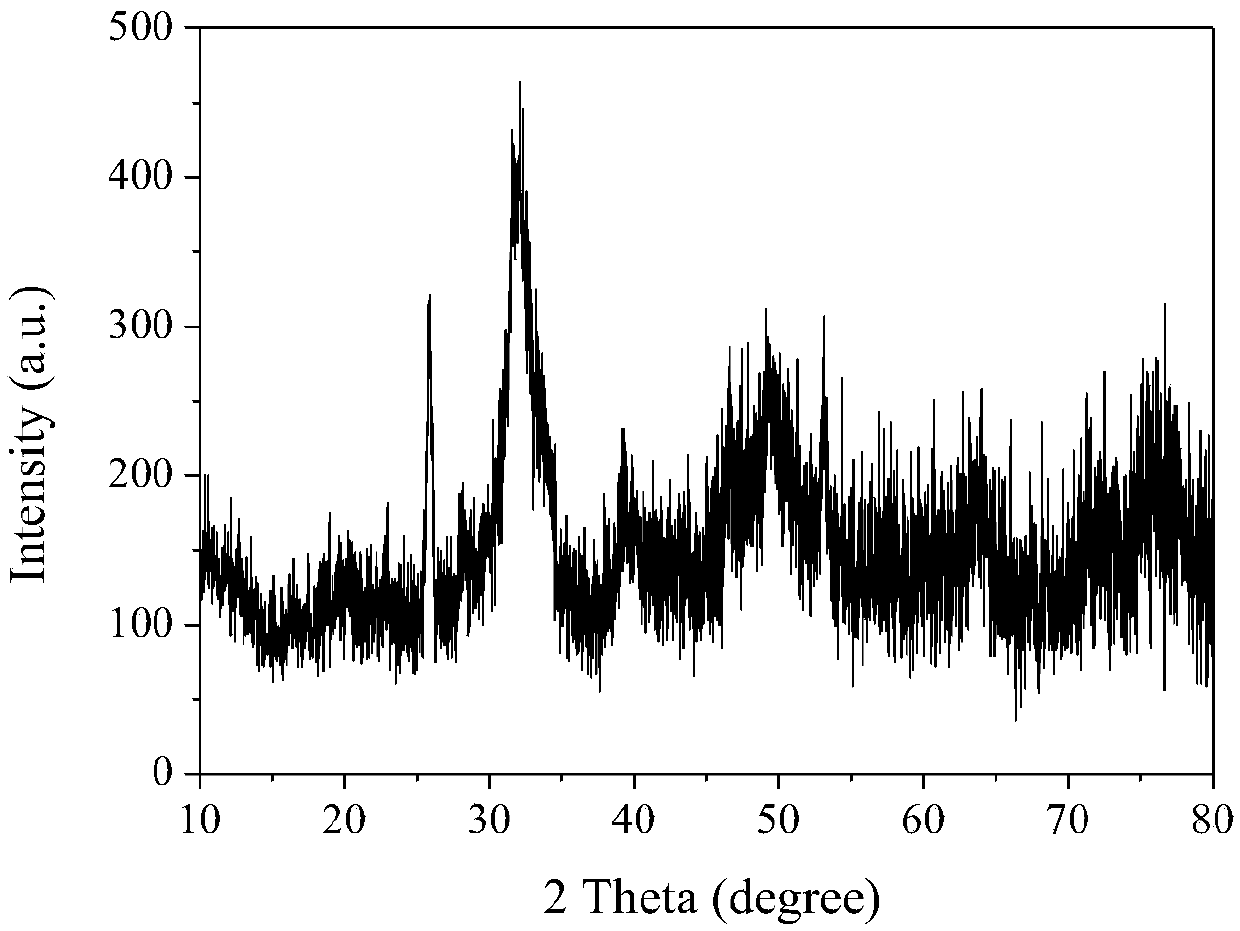
[0061] The X-ray diffraction figure of the oil-soluble hydroxyapatite nanocrystal obtained in the above...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com