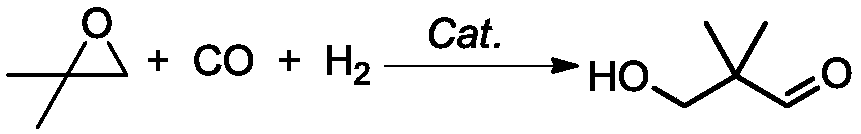Preparation method of 2,2-dimethyl-3-hydroxypropanal and application thereof in preparation of neopentyl glycol
A technology of hydroxypivalaldehyde and neopentyl glycol, which is applied in the preparation of hydroxyl compounds, the preparation of organic compounds, and the preparation of carbon monoxide reaction, etc., can solve the problem of untreated neopentyl glycol acetal compounds, NPG yield loss, influence NPG yield and other issues, to achieve the effect of effective control of raw material costs, suppression of side reactions, and cost savings of wastewater treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of HPA
[0041] Add 1kg of isobutylene oxide, 0.05g of rhodium octoate and 0.1g of boron trifluoride into a 2L pressure-resistant stirred reactor under a nitrogen atmosphere, raise the temperature to 50°C and stir for 0.5h under a nitrogen atmosphere, at this time the catalyst has completely dissolved . After the temperature of the reaction solution in the reactor was raised to 90°C, H 2 : CO molar ratio of 1:1 synthesis gas to the relative pressure of the reactor is 10MPa, when the pressure in the reactor drops, the synthesis gas is added to the system pressure of 10MPa, the temperature and pressure in the reactor are kept constant, and the gas chromatography is sampled after 6 hours of reaction Analysis showed that the conversion rate of isobutylene oxide was 99.2%, the selectivity of HPA was 91%, and the reaction was finished. After the reactor was depressurized to normal pressure, the obtained reaction solution was distilled under normal pressure, and ...
Embodiment 2
[0045] Preparation of HPA
[0046] Add 1kg of isobutane oxide, 0.5g of rhodium carbonyl and 5g of butyl titanate into a 2L pressure-resistant stirred reactor under a nitrogen atmosphere, raise the temperature to 20°C and stir for 1.5h under a nitrogen atmosphere, at which point the catalyst has completely dissolved. After the temperature of the reaction solution in the reactor was raised to 150°C, H 2 : The relative pressure of synthesis gas with CO molar ratio 1:1 to the reactor is 7MPa. When the pressure in the reactor drops, the synthesis gas is added to the system pressure of 7MPa, and the temperature and pressure in the reactor are kept constant. After 4 hours of reaction, gas chromatography is taken Analysis showed that the conversion rate of isobutylene oxide was 99.4%, the selectivity of HPA was 92%, and the reaction was finished. After the reactor was depressurized to normal pressure, the obtained reaction liquid was distilled under normal pressure, and the fraction ...
Embodiment 3
[0050] Preparation of HPA
[0051] Add 1 kg of isobutylene oxide, 10 g of cobalt carbonyl and 1 g of lanthanum trifluoromethanesulfonate to a 2L pressure-resistant stirred reactor under a nitrogen atmosphere, raise the temperature to 40°C and stir for 1 hour under a relative pressure of 0.5 MPa in a nitrogen atmosphere, and the catalyst All dissolved. After the temperature of the reaction solution in the reactor was raised to 70°C, H 2 : CO molar ratio of 1:1 synthesis gas to the relative pressure of the reactor is 20MPa, when the pressure in the reactor drops, the synthesis gas is added to the system pressure of 20MPa, the temperature and pressure in the reactor are kept constant, and the gas chromatography is sampled after 6 hours of reaction Analysis showed that the conversion rate of isobutylene oxide was 96%, the selectivity of HPA was 97.6%, and the reaction was finished. After the reactor was depressurized to normal pressure, the obtained reaction liquid was distilled...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com