Synthesis and application of colorimetric carbazole fluorescent probe for detecting hydrogen sulfide
A fluorescent probe and carbazole-based technology, applied in the field of fluorescent probes, can solve problems such as poor anti-interference ability, sample destruction, and low accuracy, and achieve the effects of low cost, low detection limit, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of embodiment 1 fluorescent probe
[0023] (1) Synthesis of 3-acetyl-N-ethylcarbazole: Dissolve 9.75g (0.05mol) N-ethylcarbazole in 150mL CH 2 Cl 2 , cooling in an ice-water bath and magnetic stirring, quickly add 8.00g (0.06mol) of anhydrous AlCl3, after stirring evenly, slowly add 5.30mL (0.075mol) of acetyl chloride dropwise, after the addition, react at room temperature for 4 hours, treat with ice water, dichloro Extract with methane, adjust the pH value of the organic layer to neutral with saturated sodium carbonate solution, then dry with anhydrous magnesium sulfate, filter with suction, and rotate the filtrate to obtain a green solid powder. The pure product was obtained by recrystallization from ethanol.
[0024] (2) Synthesis of 3-chloro-3-[3-(9-ethylcarbazolyl)]acrolein: slowly drop phosphorus oxychloride (1.395ml, 5mmol) into DMF (15ml ) solution, then remove the ice-water bath and stir for 30 minutes. 3-Acetyl-N-ethylcarbazole (1,1885 g, ...
Embodiment 2
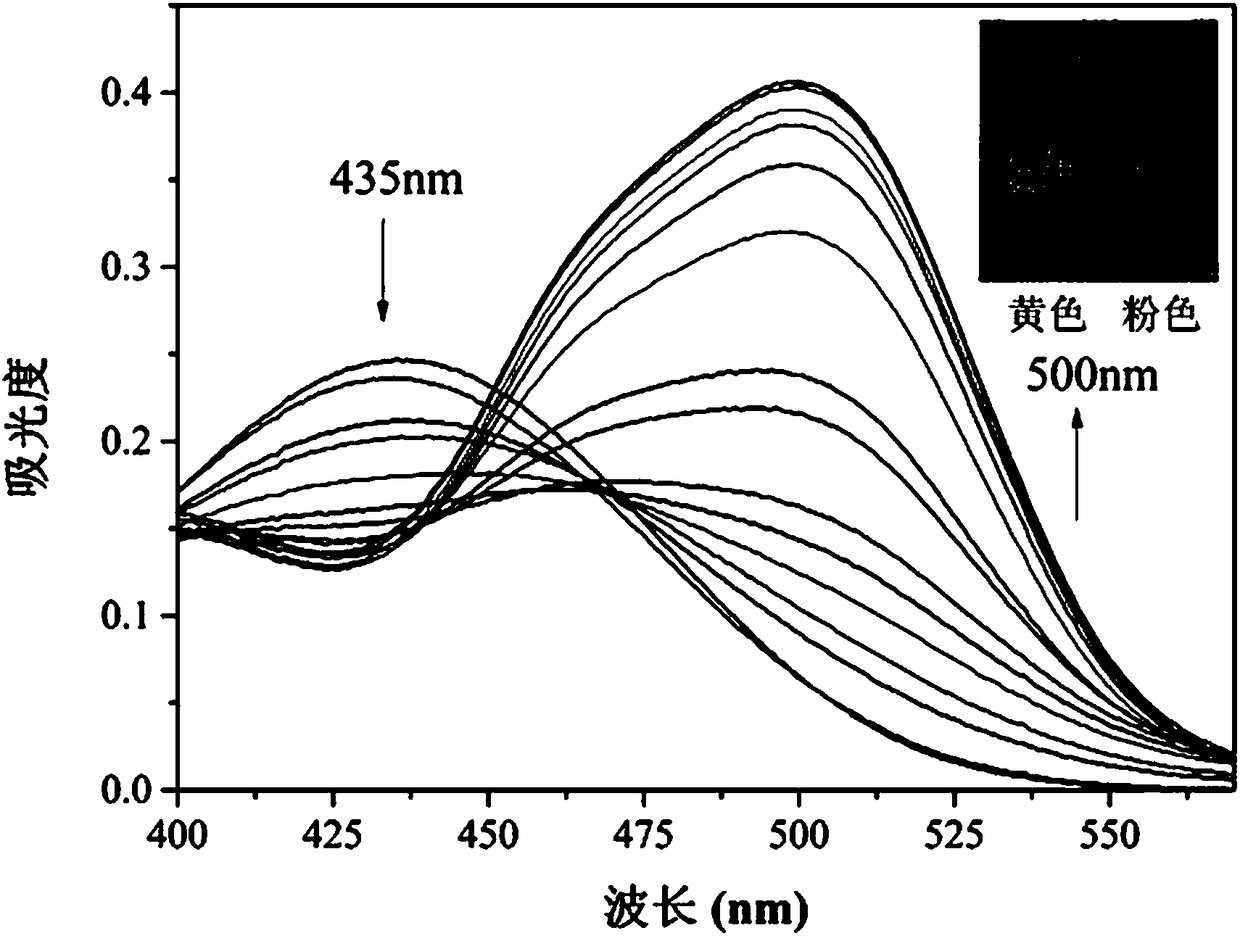
[0028] Example 2 Fluorescent probe with H 2 UV-Vis absorption spectrum of S concentration change: Add 20.0 µL fluorescent probe stock solution to 2.0 mL PBS buffer / DMSO (4 / 6, v / v, pH 7.4) system for H 2 S ultraviolet titration experiment, and record ultraviolet-visible absorption spectrum ( figure 1 ). With H 2 With the increase of S concentration, the absorption peak of the probe at 435 nm gradually decreases, and the absorption peak at 500 nm gradually increases, and the color of the solution changes from yellow to pink at the same time, indicating that the probe can realize the "naked-eye" detection of H 2 S.
Embodiment 3
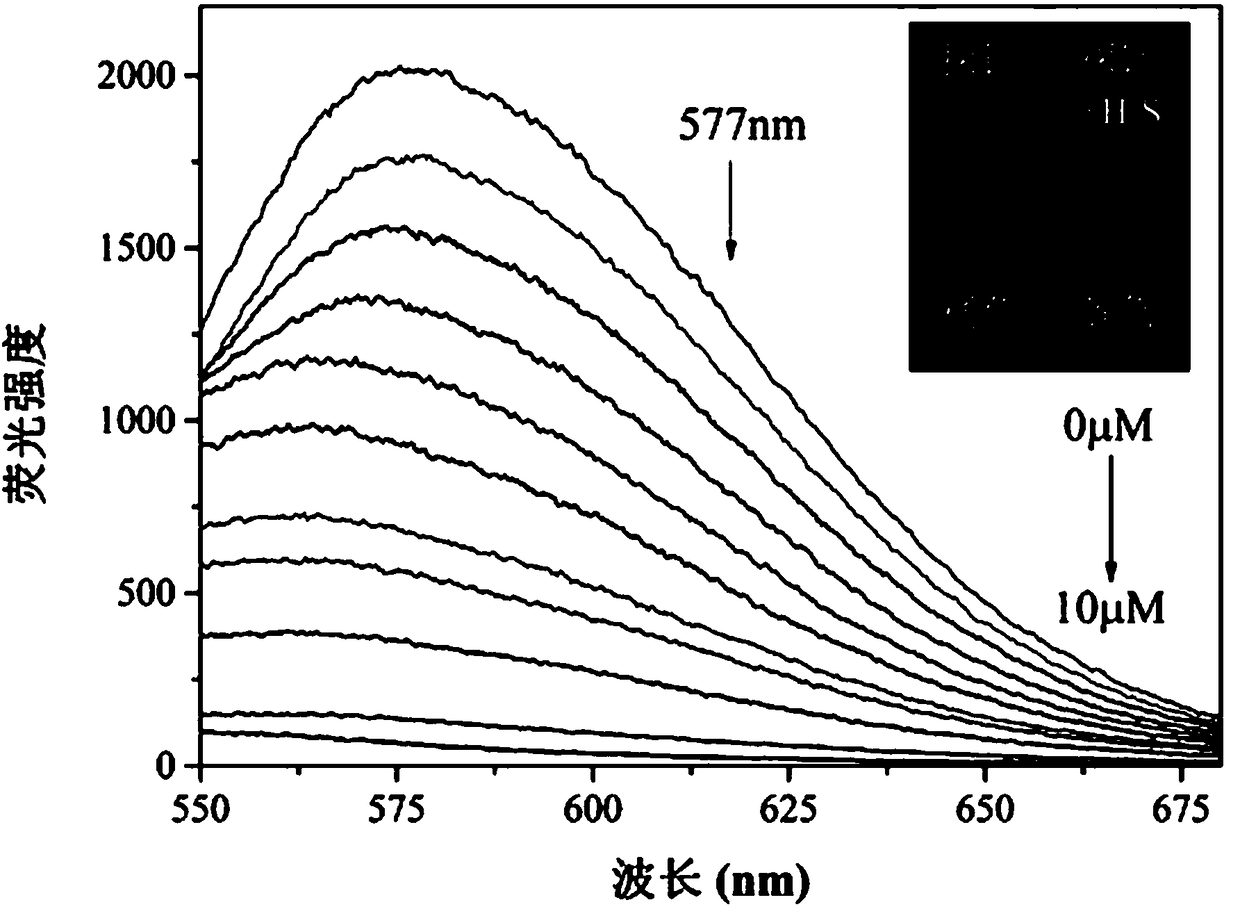
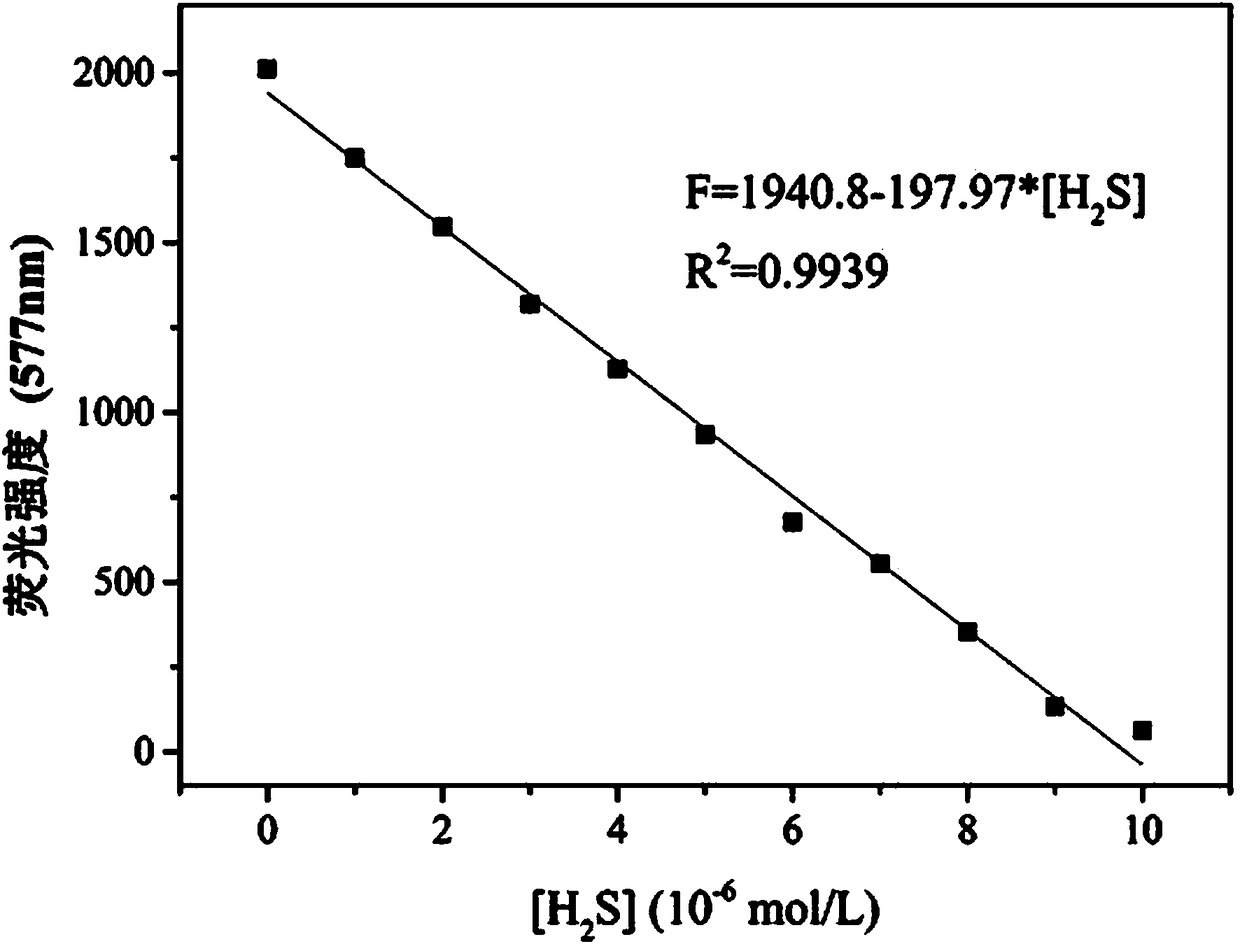
[0029] Embodiment 3 Fluorescent probe with H 2 Fluorescence emission spectrum of S concentration change: add 20.0 µL fluorescent probe stock solution to 2.0 mL PBS buffer / DMSO (4 / 6, v / v, pH 7.4) system for H 2 S Fluorescence titration experiment, detected on a fluorescence spectrophotometer. With H 2 With the increase of S concentration, the fluorescence intensity at 577 nm gradually weakened ( figure 2 ). At the same time, the fluorescence color of the solution changed from orange to blue under the irradiation of 360nm ultraviolet lamp. Instrument parameters: the slit widths of the excitation wavelength and emission wavelength are 10 nm and 10 nm respectively, the voltage is 600 V, and the maximum excitation wavelength of the fluorescent probe solution is: λ ex 445 nm and a maximum emission wavelength of λ em 577nm. with H 2 S concentration is the abscissa, and the fluorescence intensity at 577 nm is the ordinate to draw a graph to obtain H 2 The working curve of S ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com