Method for using sulphur gas for reducing waste sulfuric acid to prepare liquid sulfur dioxide and sulfuric acid
A liquid sulfur dioxide and sulfur dioxide technology, applied in chemical instruments and methods, sulfite preparation, sulfur compounds, etc., can solve problems such as high power consumption, high investment, and affecting the quality of finished acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
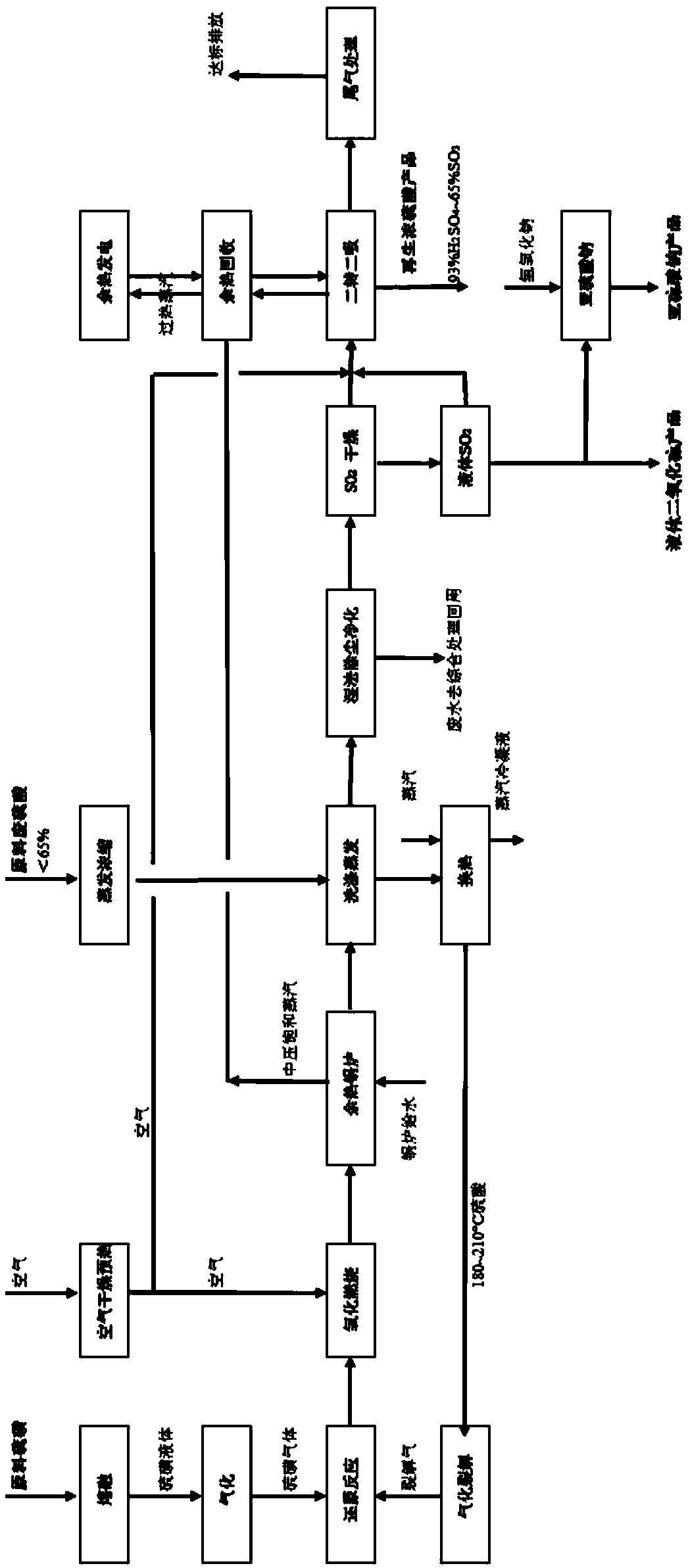
[0084] Such as figure 1 As shown, the waste sulfuric acid w(H 2 SO 4 )50~52%, organic matter content w5~8%, water content 35~40%, raw material quantity 50t / h, after being pumped, self-cleaning and filtered, then sent to evaporate and concentrate to w(H 2 SO 4 ) 65%, and then pumped into the first washing tower of the purification and dust removal system, after being circulated and washed with the hot furnace gas from the waste heat (boiler), the temperature rises to 160-180°C, and the concentration rises to w(H 2 SO 4 ) to 73-75%, and then pump the preheated waste sulfuric acid to the heat exchanger, heat it to 190-200°C with the by-product steam of the waste heat recovery system, and then enter the sulfuric acid gasification and cracking furnace, and maintain the temperature of the cracking furnace through the electric heater The temperature is 500-650°C. The sulfuric acid in the waste sulfuric acid is quickly gasified into sulfur trioxide gas and water vapor, and the liq...
Embodiment 2
[0092] As shown in reference 1, the alkylation by-product waste acid, the main component is w(H 2 SO 4)84~86%, hydrocarbon mass fraction 8~12%, raw material amount 120t / h, temperature ~30℃ raw material waste sulfuric acid is sent to waste sulfuric acid regeneration through pump and self-cleaning filter to remove mechanical debris and large particle solids The treatment device, after directly washing and exchanging heat with the hot furnace gas from the waste heat boiler, the temperature rises to 160°C, and then is heated to 210-230°C by the by-product steam of the waste heat recovery system to approach the gasification point, and then enters sulfuric acid gasification and cracking The temperature of the gasification and cracking furnace is maintained at 450-650°C by an electric heater. The sulfuric acid in the waste sulfuric acid is quickly gasified and decomposed into sulfur trioxide gas and water vapor, and the liquid organic matter is gasified and cracked into small molecul...
Embodiment 3
[0099] Get 15.0t / h of the liquid sulfur dioxide product in Example 2, adopt w(NaOH)30% sodium hydroxide solution~66t / h to wash and absorb after heating and vaporizing, and concentrate to w(NaOH) through spray evaporation 2 SO 3 ) 63~65%, decrystallization and drying to get anhydrous sodium sulfite product, product sodium sulfite purity w(Na 2 SO 3 )>97.2%, iron w(Fe)<0.003%, water insoluble matter ≤0.02%, sulfate ≤2.2%, chloride ≤0.08%, in line with national standards: anhydrous sodium sulfite standard (HG / T2967-2010) middle class product quality requirements.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com