Lithium sulfur battery cathode material and preparation method thereof
A positive electrode material, lithium-sulfur battery technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of limiting battery life, reducing Coulombic efficiency, poor conductivity of active materials, etc., to prolong battery life and alleviate volume effect , the effect of maintaining Coulombic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The above-mentioned porous basic manganese oxide can be obtained by referring to the following preparation method, specifically, the method is a hydrothermal method, including the following steps:
[0062] (1) preparing 1% to 50% manganese salt aqueous solution, wherein the manganese salt is at least one of manganese nitrate, manganese sulfate, manganese chloride and manganese bromide;
[0063] (2) Optionally, add a small amount of alkaline solution to the manganese salt solution described in step (1), and stir to obtain mixture A;
[0064] (3) The manganese salt solution of step (1) or the mixture A of step (2) is subjected to a hydrothermal reaction at 120-220°C for 6-24h, and the product after the reaction is subjected to solid-liquid separation, and the obtained solid is Vacuum drying at 100°C yields porous basic manganese oxide.
[0065] Wherein, the manganese salt in step (1) is preferably manganese nitrate, and the alkaline solution of step (2) can be the aqueou...
Embodiment 1
[0101] Take 70ml of 20wt% manganese nitrate aqueous solution, add a few drops of ammonia water and stir evenly, transfer it into a 100ml hydrothermal reaction kettle, raise the temperature of the reaction system to 140°C under airtight conditions, and carry out hydrothermal reaction for 12h. After the reaction is complete, cool under natural conditions. The hydrothermal product was separated by suction filtration, washed several times with distilled water, and dried in vacuum at 70° C. for 12 hours to obtain porous basic manganese oxide in the form of light brown powder.
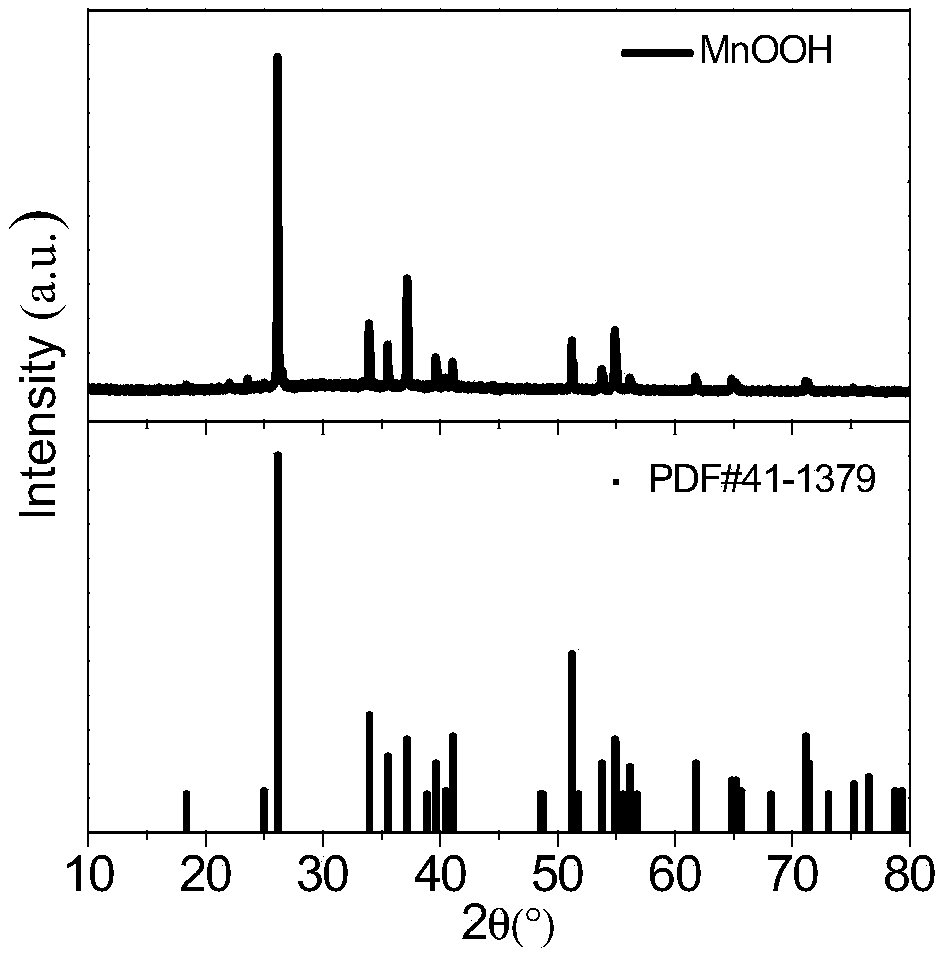
[0102] The porous basic manganese oxide prepared in this example 1 is subjected to morphology analysis, and the obtained spectrum is shown in Figure 1-3 .
[0103] figure 1 Shown is the X-ray diffraction (XRD) collection of illustrative plates (collection number MnOOH) of the porous basic manganese oxide prepared in this embodiment 1, and the present invention provides the XRD collection of porous basic m...
Embodiment 2
[0107] The porous basic manganese oxide material prepared in Example 1 was mixed with conductive carbon black and PVDF binder in a ratio of 8:1:1, scraped and coated on copper foil, and dried at 70°C to replace lithium metal As the negative electrode, the secondary electrolyte LB-114 was selected as the lithium ion electrolyte to test the performance of the lithium battery positive electrode material prepared by porous basic manganese oxide. The capacity decreased from 440mAh / g to 338mAh / g, and the specific capacity retention rate was 77%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Membrane resistance | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com