Easily-modified near-infrared region II organic small molecule dye and synthetic method and application thereof
A near-infrared, small molecule technology, applied in the field of bioluminescence imaging, which can solve the problems of low yield and difficult synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
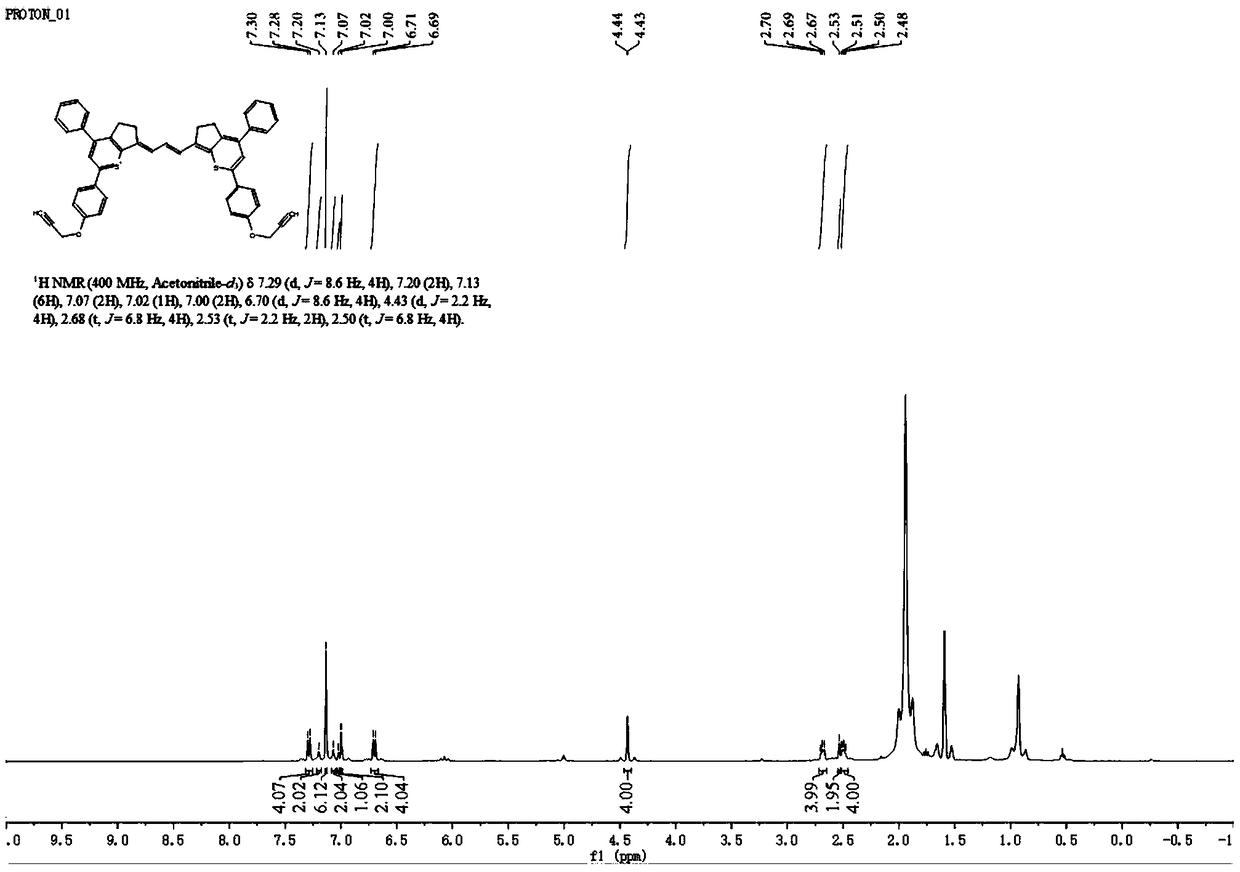
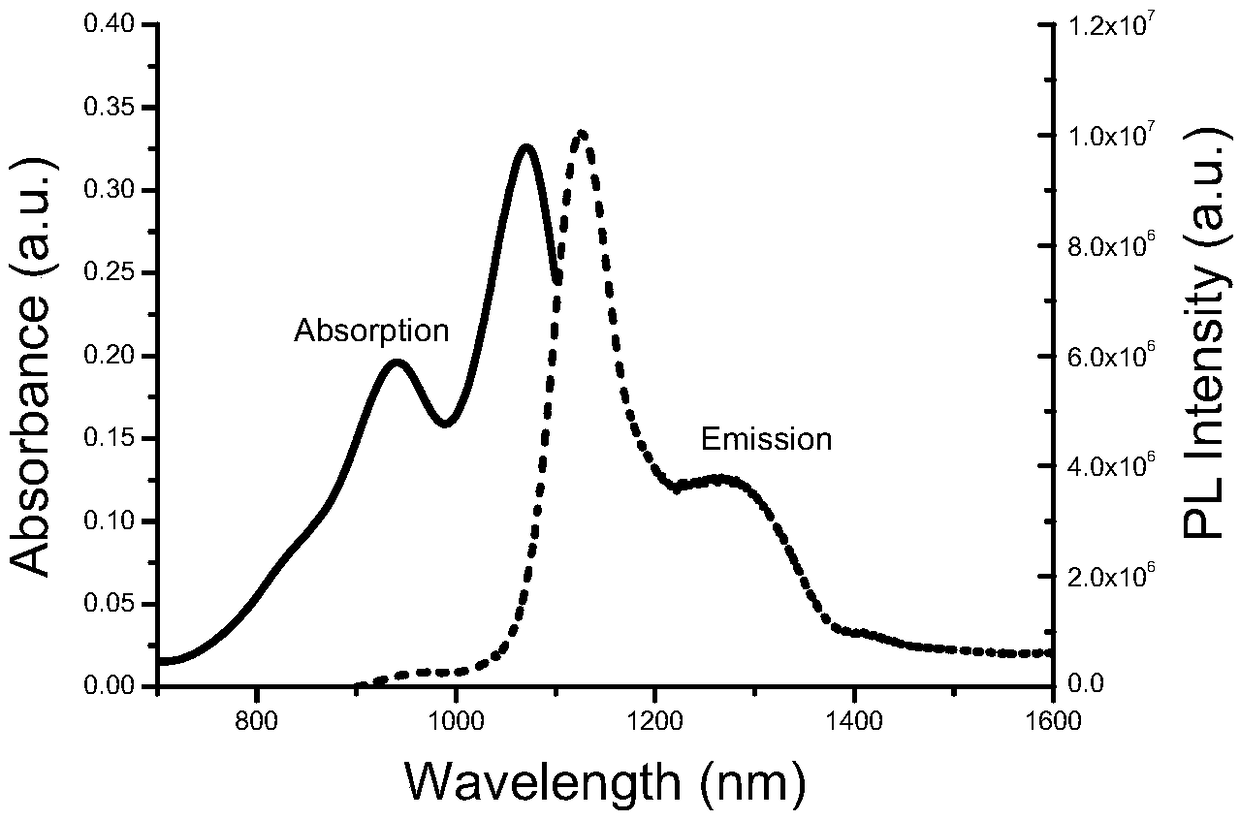
[0070] The synthetic route of the small molecule dye 5H5 in the second near-infrared region is as follows:
[0071]
[0072]
[0073] 1) In a 500mL round bottom flask, dissolve p-hydroxyacetophenone (100mmol, 13.6g) in 200mL of methanol, add potassium hydroxide solution (50%W / W, 70mL) under ice-water bath conditions at 0°C, stir and mix Mix well to fully dissolve. Another benzaldehyde (100 mmol, 10.6 g) was dissolved in 30 mL of methanol, placed in a constant pressure dropping funnel and added dropwise to the above reaction solution. Stir at 0°C for 1 h, then turn to room temperature and stir the reaction overnight. The reaction was stopped, methanol was removed using a rotary evaporator, and 3M hydrochloric acid solution was added dropwise to the remaining solution until pH = 7, and a large amount of yellow solid was precipitated. The filter residue was collected by filtration, and recrystallized from methanol to obtain 18.8 g of light yellow crystals, which were 1-(4...
Embodiment 2
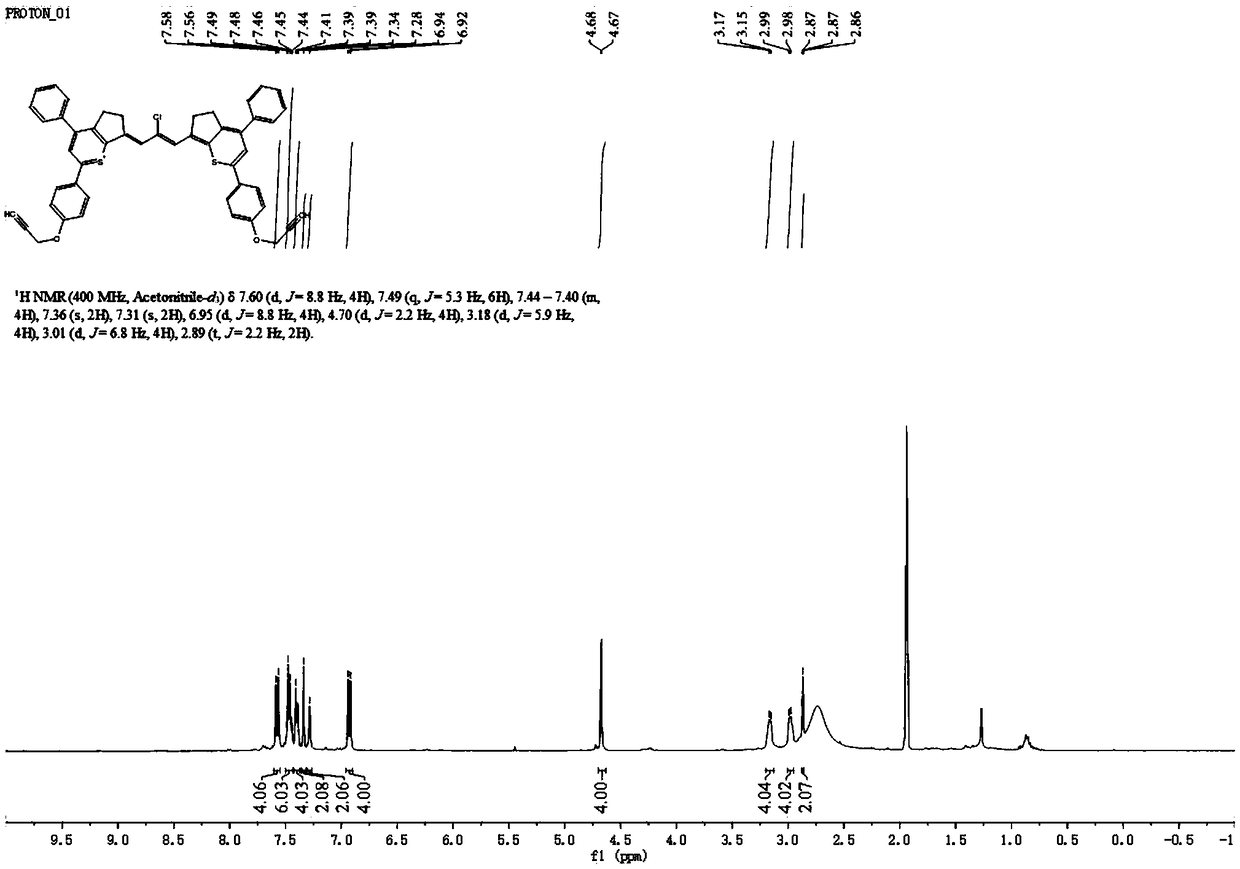
[0085] The synthetic route of the small molecule dye 5L5 in the second near-infrared region is as follows:
[0086]
[0087] 1) In a 100mL round bottom flask, add furochloric acid (10mmol, 1.68g) dissolved in 10mL ethanol. Aniline (22mmol, 2mL) was dissolved in 10mL of ethanol, dropped into the above solution through a constant pressure dropping funnel within 0.5h, and stirred at room temperature for 5h. The reaction solution was concentrated by rotary evaporation to 5 mL, and then poured into 100 mL of diethyl ether, a large amount of golden solid was precipitated, filtered, washed twice with 10 mL of ice ethanol, the filter residue was collected, and dried in vacuo to obtain 1.6 g of golden solid, which was compound 5b. Yield 62%.
[0088] 2) Compound 4a (0.02 mmol, 8.6 mg), compound 5b (0.01 mmol, 2.9 mg) and sodium acetate (0.02 mmol, 1.6 mg) were dissolved in 1 mL of acetic anhydride and reacted at 70°C for 2 h. After cooling to room temperature, the reaction solutio...
Embodiment 3
[0095] The synthesis route of the small molecule dye 6H6 in the second near-infrared region is as follows:
[0096]
[0097] 1) Dissolve cyclohexanone (10mmol, 1.03mL) and tetrahydropyrrole (10mmol, 0.84mL) in 20mL of benzene in a 25mL round bottom flask, connect it to a Dean-Stark trap, and reflux for 4 hours. The solvent was removed by rotary evaporation, the residue was dissolved in 1,4-dioxane, compound 2 (5 mmol, 1.31 g) was added, and the reaction was refluxed for 2 h. The reaction was cooled to room temperature, added water 30 mL, extracted with ethyl acetate (3x10 mL). The organic phases were combined and dried over anhydrous sodium sulfate. Spin dry and pass through a column (200-300 mesh silica gel column, n-hexane / ethyl acetate, 5 / 1, v / v). 1.23 g of compound 3b was obtained as a white solid. Yield 68%. Further HPLC preparation separated and purified two isomers, which were only used for NMR characterization.
[0098] 2) Dissolve compound 3b (2mmol, 0.36g) in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com