A kind of cytoglobin-human lactoferrin peptide fusion protein, gene and application
A technology of fusion protein and lactoferrin, applied in the biological field, can solve problems such as unhelpful product functions, and achieve the effects of strong antibacterial effect, increased storage stability, and convenient purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Small amount of expression of TAT-CYGB-hLFcin in Escherichia coli
[0029] (1) Cell penetrating peptide TAT (SEQ NO.3), cytoglobin Cytoglobin (SEQ NO.4), and human lactoferrin peptide (SEQ NO.5) are sequentially connected in series to form cytoglobin-human lactoferrin peptide hybrid protein. The TAT-CYGB-hLFcin hybrid protein gene coding sequence was handed over to Gene Synthesis Company for whole gene synthesis through codon optimization software.
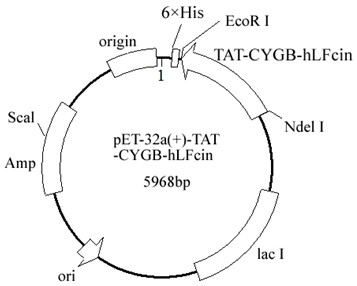
[0030] (2) Design full-length splicing primers to insert the TAT-CYGB-hLFcin gene into the expression vector pET32a(+) through the restriction sites Nde I and EcoR I ( figure 1 ), the accuracy of the final expression vector was confirmed by enzyme digestion and sequencing, and finally transferred to E. coli BL21 expression strains to obtain engineering strains containing TAT-CYGB-hLFcin fusion protein. Take 100 μL of the recombinant bacteria and evenly spread it on the LB plate (containing kanamycin sulfate at a...
Embodiment 2
[0032] Example 2 Separation and purification of TAT-CYGB-hLFcin fusion protein
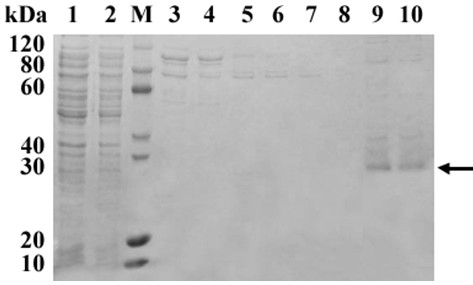
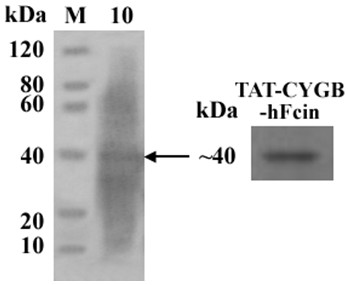
[0033] (1) Collect the whole fermented bacteria containing supernatant, centrifuge to obtain the supernatant, resuspend in 20 mM Tris-HCl (pH 8.0) and centrifuge to obtain the protein supernatant, through cation exchange (CM Sepharose Fast Flow) chromatography, S -100 molecular sieve chromatography and Sephadex G-25 gel filtration chromatography desalted to obtain TAT-CYGB protein, SDS-PAGE electrophoresis detection, Western blotting qualitative inspection CYGB protein.
[0034] (2) CM cation exchange column: the column volume is 20mL, the UV detection wavelength is 220~280nm, and the column is equilibrated with 20mM Tris-HCl buffer (pH 8.0) at a flow rate of 1.5mL / min; The buffer was used to equilibrate the column again, and then the above buffer (pH 8.0) containing 500mM NaCl was used as the eluent for elution at a flow rate of 1.5mL / min. After collecting all the elution peak samples, all the c...
Embodiment 4
[0038] Example 4 Research on the Antibacterial Spectrum of TAT-CYGB-hLFcin Fusion Protein
[0039] (1) Detect the antibacterial activity of the fusion protein samples collected in step (5) of Example 2 against the strains listed in Table 1. Various bacteria were cultured in 5mL liquid medium shaker culture, and the culture conditions are listed in Table 1. When the bacterium grows to logarithmic growth phase, carry out the mensuration of inhibition zone according to the method of embodiment 1 method respectively. Measured 3 times to take the average value.
[0040] (2) Medium (g / L) composition. ① LB medium: tryptone 10g, yeast extract powder 5g, NaCl 10g, deionized water 1L, pH natural; ② Cha's medium: sucrose 30g, sodium nitrate 3g, dipotassium hydrogen phosphate 1g, magnesium sulfate 0.5g, chlorine Potassium chloride 0.5g, ferrous sulfate 0.01g, deionized water 1L, pH natural; ③Nutritional agar: beef extract 3g, peptone 10g, NaCl 5g, agar 20g, deionized water 1L, pH 7.0; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com