Macrolide and carbostyril heterocomplex and preparation method thereof
A macrolide and quinolone technology, applied in the preparation of macrolide and quinolone hybrids, in the field of macrolide and quinolone hybrids, can solve the problems of low activity of Streptococcus pneumoniae and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
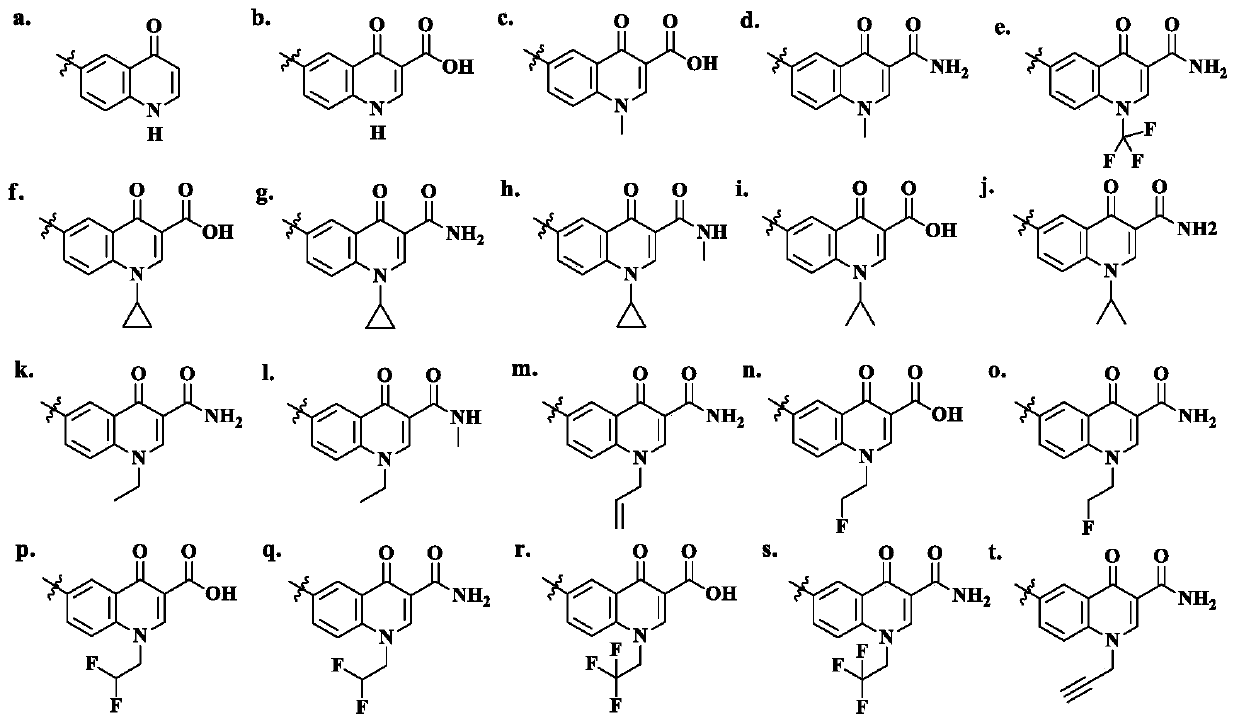
[0024] The present invention provides a hybrid of macrolide and quinolone, the hybrid of macrolide and quinolone is a compound with general formula I and formula II, or, the hybrid of macrolide and quinolone Compounds include pharmaceutically acceptable salts formed by compounds of the general formula I and II and inorganic acids or organic acids, which are metabolized or converted into the general structure as prodrugs under physiological conditions in vivo. Shown compound, and plays a pharmacological role as an active ingredient. In the embodiments of the present invention, various pharmaceutically acceptable acids can form salts on the nitrogen of 5-O-desosamine dimethylamino in general formula I and general formula II, and the prodrug is esterified on the 2'-OH , similar as erythromycin ethyl succinate as a prodrug, the ester group can be hydrolyzed in vivo to release 2'-OH again. Conventional methods for the preparation of prodrugs can be found in Design of Prodrugs (H. ...
Embodiment 2
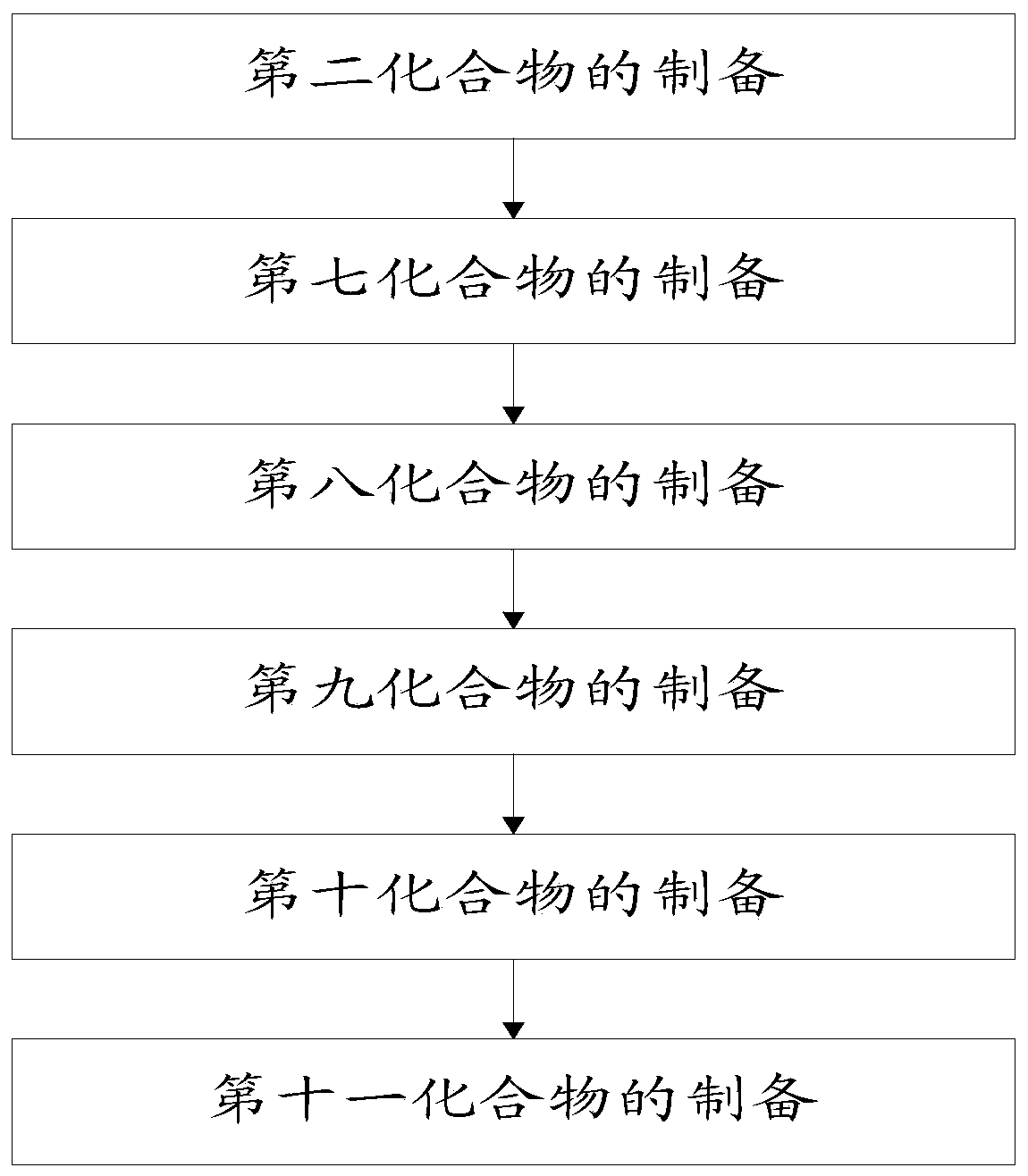
[0081] The reaction scheme of a kind of macrolide and quinolone hybrid in the embodiment of the present invention is as follows, and described macrolide and quinolone hybrid are the compound with general formula II, prepare as shown in general formula II The method of described macrolide and quinolone hybrid specifically can comprise the following steps:
[0082]
[0083] The reaction conditions are: (a) acetic anhydride, methylene chloride; (b) potassium tert-butoxide, 80% propargyl bromide, dimethyl sulfoxide / tetrahydrofuran (1:1); (c) pyridine, triphosgene, Dichloromethane, -10℃-room temperature; (d) N-chlorosuccinimide (NCS), dimethyl sulfide (DMS), triethylamine, dichloromethane, -15--5℃; (e ) N-fluorobenzenesulfonimide (NFSI), NaH, N, N-dimethylformamide, -5-0°C; (f) ArBr or ArI, CuI, bis(triphenylphosphine) dichloride Palladium chloride, triethylamine, 80°C, acetonitrile; (g) 65°C, methanol.
[0084] In order to enable those skilled in the art to better understand ...
Embodiment 3
[0140] The reaction scheme of a kind of macrolide and quinolone hybrid in the embodiment of the present invention is as follows, and described macrolide and quinolone hybrid are the compound with general formula I, prepare as shown in general formula I The method of described macrolide and quinolone hybrid specifically can comprise the following steps:
[0141]
[0142] The reaction conditions are: (a) potassium tert-butoxide, allyl bromide, dimethyl sulfoxide / tetrahydrofuran (1:1); (b) pyridine, triphosgene, dichloromethane, -10°C-room temperature; (c ) N-chlorosuccinimide (NCS), dimethyl sulfide (DMS), triethylamine, dichloromethane, -15--5 ° C; (d) N-fluorobenzenesulfonimide (NFSI ), NaH, N, N-dimethylformamide, -5-0°C; (e) ArBr or ArI, palladium acetate, tris(o-methylphenyl)phosphorus, triethylamine, 60-90°C, acetonitrile ; (f) 65°C, methanol.
[0143] In order to enable those skilled in the art to better understand the present invention, the preparation method of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com