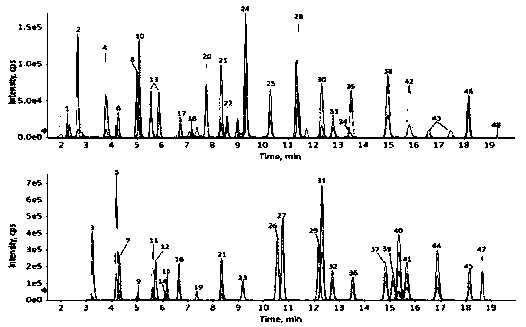
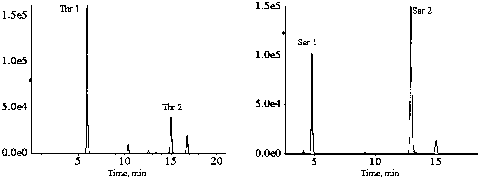
Method of rapidly and quantitatively analyzing 48 amino acids
An amino acid, quantitative ion technology, applied in analytical materials, measuring devices, material separation, etc., can solve the problems of many test steps, high price, poor resistance to matrix interference, etc., to improve extraction efficiency, reduce analysis costs, and improve methods. The effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Example 1 A method for rapid quantitative analysis of 48 amino acids
[0114] 1. Reagents
[0115]Standards of pipemidic acid (purity≥97.0%) and α-aminobutyric acid (purity≥99.0%) were purchased from Anpu Company (Shanghai, China), and other standard amino acids, trihydroxypropylphosphine and ammonium formate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Chloroformyl propyl ester, 3-picoline, isooctane and n-propanol were purchased from Tokyo Chemical Industry Co. (Tokyo, Japan). 13 C 3 - Propanol, D 3 -Methionine and D 4 - Cystathionine was purchased from Cambridge Isotope Laboratories (Andover, MA, USA).
[0116] Solution preparation
[0117] Amino acid standard stock solution: Accurately weigh each amino acid standard, dissolve with ultrapure water or 1mol / L HCl solution to volume.
[0118] Standard 1: Accurately weigh the amino acid standard as shown in Table 2, add water or 1mL of 1mol / L hydrochloric acid aqueous solution to dissolve, and prepare a...
Embodiment 2
[0163] Example 2 A kit for detecting amino acid content by derivatization HPLC-MS / MS method
[0164] 1. Composition
[0165] Derivatization reagent kits, internal standard kits, and standards
[0166] Wherein, the combination of derivatization reagents is: derivatization reagent A and derivatization reagent B prepared in Example 1;
[0167] The combination of internal standard solution is: internal standard solution A and internal standard solution B prepared in Example 1;
[0168] The standard products are: standard product 1, standard product 2 and standard product 3.
[0169] 2. How to use
[0170] (1) Preparation of calibrator
[0171] According to the method of Example 1, calibrator 1 to calibrator 7 were prepared.
[0172] (2) Sample pretreatment
[0173] With embodiment 1.
[0174] (3) Sample data collection
[0175] With embodiment 1.
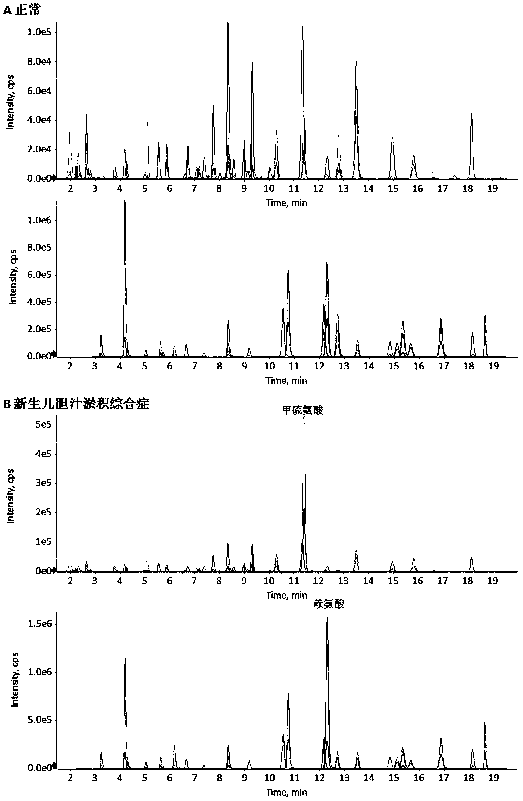
Embodiment 4
[0176] Embodiment 4 detects clinical sample
[0177] 1. Test samples
[0178] The plasma of volunteers without abnormal amino acid metabolism and patients with neonatal cholestasis syndrome was selected for detection.
[0179] 2. Detection method
[0180] As described in Example 1.
[0181] 3. Test results
[0182] Test results such as figure 2 shown. The content of methionine, citrulline and lysine in the plasma of patients with neonatal cholestasis syndrome was significantly increased, and the plasma amino acid profile of volunteers without abnormal amino acid metabolism was normal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com