Preparation and application of medicine-carrying polydopamine/dendrimer-gold nanoparticles
A technology of polydopamine nano-dendrimer, which can be applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and photodisintegration of drugs in the body, and can solve problems that have not been fully explored , to achieve the effect of improving the effect of chemotherapy, reducing drug resistance and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Take 2.6 mL, 90 mL, and 40 mL of ammonia water, ethanol, and distilled water respectively, and magnetically stir them in a 45°C water bath to fully mix them. After 15 minutes, 10 mL of 50 mg / mL dopamine monomer aqueous solution was added. After reacting for 12 h, centrifuge and purify (16000 rpm, 10 min) to collect polydopamine nanoparticles.
[0046] According to the molar ratio of 1:50, the dendrimer and the chloroauric acid aqueous solution were mixed, stirred and mixed at room temperature for 15 minutes. Then, according to the molar ratio of 1:3, sodium borohydride aqueous solution was added, and the reaction was stirred for 2 hours. After the reaction, the solution was dialyzed for 2 days (6 times, 1 L / time), and freeze-dried to obtain the dendrimer-gold nanoparticles.
[0047] According to the mass ratio of 1:0.5, the polydopamine nanoparticles were slowly added dropwise into the dendrimer-gold nanoparticles, and the reaction solution was 10 mM Tris buffer with ...
Embodiment 2
[0051] Prepare an ethanol solution of doxorubicin hydrochloride, and add it to the aqueous solution of PDA-G5Au-PEG nanoparticles prepared in Example 1 at a mass ratio of 1:1. After stirring and mixing, triethylamine was added. After 24 hours of reaction, the drug-loaded polydopamine / dendrimer-gold (PDA-G5Au-PEG@DOX) composite nanoparticles were obtained by centrifugation and water washing purification.
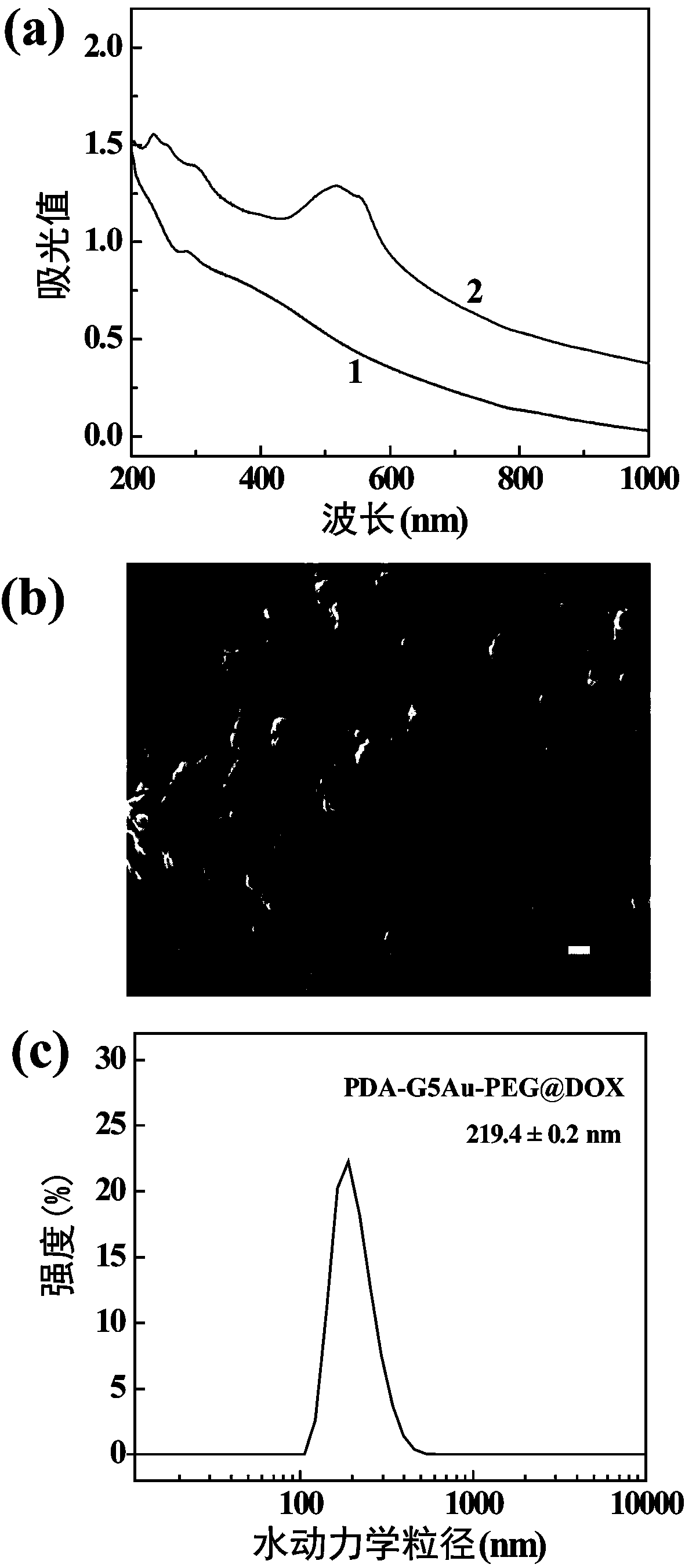
[0052] UV-Vis spectrum display (attached figure 2 a), There is an obvious absorption peak around 480 nm, indicating the successful loading of doxorubicin. Field emission electron microscope photos show (attached figure 2 b) shows that the morphology of PDA-G5Au-PEG@DOX nanoparticles is relatively uniform. The test results of laser particle size analyzer (DLS) showed the hydrodynamic size and uniformity of the obtained composite nanoparticles. Refer to the attached figure 2 c. The average size of PDA-G5Au-PEG@DOX composite nanoparticles is 219.4 ± 0.2 nm.
Embodiment 3
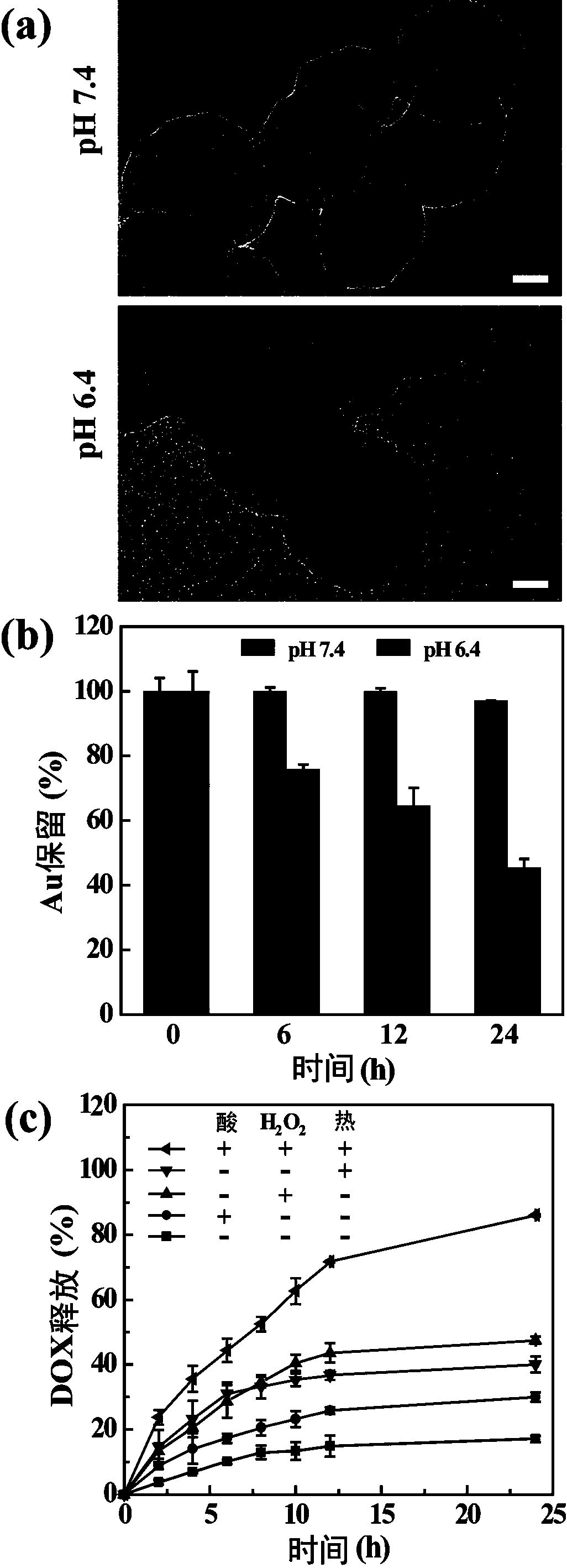
[0054] Take the PDA-G5Au nanoparticles prepared in Example 1, put them in the buffer solution of pH 7.4 and pH 6.4, place them on a constant temperature shaker at 37°C, and take samples after 0, 6, 12, and 24 hours respectively, and collect the products. PDA-G5Au-PEG@DOX nanoparticles were placed under different conditions (pH7.4 buffer, pH6.4 buffer, 20 mM hydrogen peroxide, 1.75 W / cm 2 laser irradiation), placed in a constant temperature shaker, and samples were taken regularly to study the release of the chemotherapy drug DOX.
[0055] Transmission electron microscope photos show (attached image 3 a), After shaking for 24 hours, the small black particles on the PDA "core" at pH 6.4 were significantly less than those at pH 7.4. This suggests that mildly acidic conditions can promote the release of "satellite" G5Au nanoparticles. Inductively coupled plasma optical emission spectroscopy (ICP-OES) test analysis (attached image 3 b) It was found that under the condition of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| release amount | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com