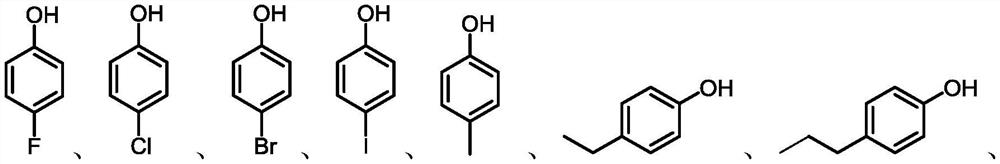
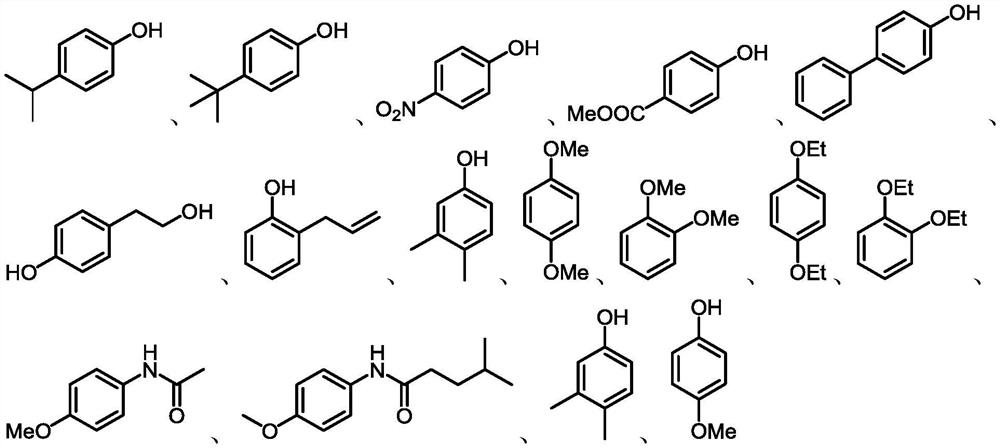
A kind of nitration method of aryl phenols or aryl ether derivatives
A technology of derivatives and aryl ethers, applied in the field of organic synthesis, can solve the problems of many by-products, many by-products of amplified reaction, safety risks, etc., and achieve the effects of being beneficial to industrial production, simplifying production processes, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] React according to the following reaction equation:
[0046]
[0047] Substrate:; Target product: (C 8 H 9 NO 4 );
[0048] The specific operation steps are as follows: p-xylylene ether (100 mmol), TMSCl (2 equiv, 200 mmol), guanidine nitrate (1.4 equiv, 140 mmol), copper sulfate pentahydrate (0.1 equiv, 10 mmol) and acetonitrile (100 mL) were added to 250 mL. in a round-bottomed flask. The reaction was left to stir homogeneously at room temperature for 12 hours and monitored by TLC. After the reaction was completed, it was filtered, and then the solvent was removed with a rotary evaporator and purified by column chromatography on silica gel (200 mesh) using petroleum ether (PE) / ethyl acetate (EA) as eluent. The result is 2-nitro-1,4-dimethoxybenzene.
[0049] Yellow solid: Yield = 96%, 1 H NMR (400MHz, Chloroform-d) δ.39 (dd, J=3.2, 1.6Hz, 1H), 7.11 (ddd, J=9.2, 3.1, 1.5Hz, 1H), 7.03 (dd, J=9.2, 1.4 Hz, 1H), 3.91(s, 3H), 3.81(s, 3H).
Embodiment 2-19
[0050] Embodiment 2-19 all reacts according to following reaction equation:
[0051]
[0052] The specific operation steps are as follows: Substrate (0.5 mmol), TMSCl (2 equiv, 1 mmol), guanidine nitrate (1.4 equiv, 0.7 mmol), copper sulfate pentahydrate (0.1 equiv, 0.05 mmol) and acetonitrile (3 mL) were added to 10 mL Snake Tube. The reaction was left to stir homogeneously at room temperature for 2-24 hours and monitored by TLC. After the reaction was completed, the solvent was removed with a rotary evaporator, and column chromatography was performed on silica gel (200 mesh) using petroleum ether (PE) / ethyl acetate (EA) as eluent.
Embodiment 2
[0054] Substrate: Target product: (C 6 H 4 FNO 3 );
[0055] Yellow solid: Yield = 57%, 1 H NMR(400MHz, Chloroform-d)δ10.36(s,1H),7.82(dd,J=8.1,3.1Hz,1H),7.36(ddd,J=9.3,7.2,3.1Hz,1H),7.16( dd, J=9.2, 4.6 Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com