Catalyst for preparing 3, 4-dichlorobenzonitrile and preparation method and application thereof
A technology of dichlorobenzonitrile and catalyst, applied in chemical instruments and methods, chemical elements of heterogeneous catalysts, catalysts for physical/chemical processes, etc., can solve problems such as short lifespan, long reaction route, and difficulty in industrialized production. , to achieve the effect of improving strength and wear resistance, improving activity and selectivity, and prolonging service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Dissolve 10g of urea in 20mL of water and mix evenly with 80mL of 30% silica sol; 15.73g of H 2 C 2 o 4 2H 2 O was dissolved in 100 mL of 80°C distilled water, and 7.57 g of V was added 2 o 5 React until no gas is produced, then add 18.63 grams of HSb(OH) 6 , 5.75 grams of 85% H 3 PO 4 , 2.58 g H 3 BO 3 , 4.84 g Co(NO 3 ) 2 ·6H 2 O and 0.09 g Na 2 CO 3 , after forming a homogeneous solution, slowly add to the above-mentioned urea-dissolved silica sol mixed solution. After stirring evenly, the catalyst precursor was obtained by high-speed spray centrifugal drying, dried in a muffle furnace at 110° C., and then gradually raised to 550° C. for 6 hours. After natural cooling, set aside. The active composition of the catalyst is: VSb 1 P 0.6 B 0.5 co 0.2 Na 0.02 o 7.46 ; The weight percentage of carbon nitride in the silica gel-carbon nitride composite carrier is about 20%. The average particle size of the catalyst is 53μm, the bulk density is 1.02g / ml,...
Embodiment 3~8
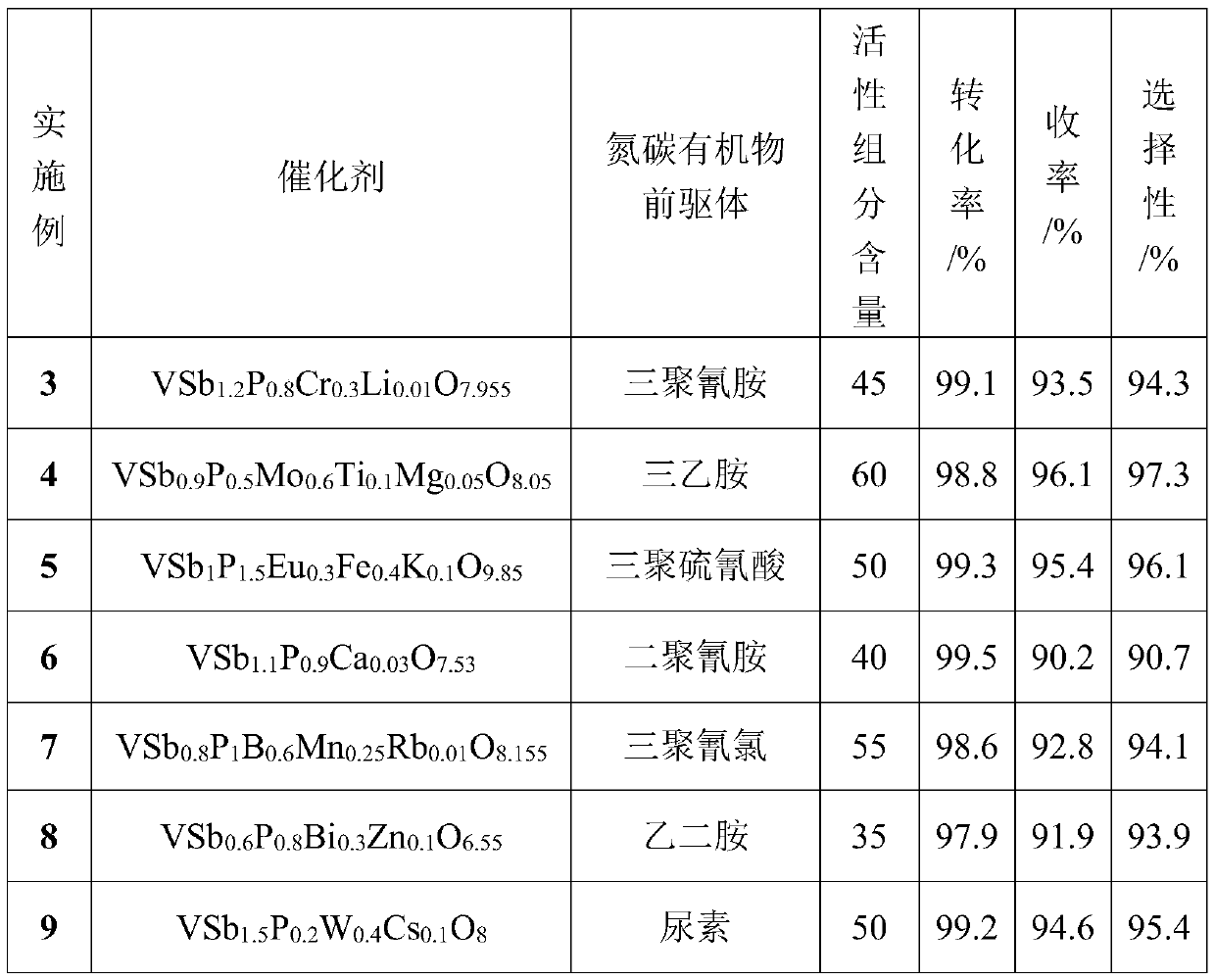
[0027] Catalyst formula is different, and reaction condition is with embodiment 1, and result is as follows:
[0028]
[0029] The catalyst of the present invention containing other co-catalysts can be prepared according to the above method, wherein the corresponding precursors can be oxides, salts, acids or bases of other co-catalyst components. During the preparation, the desired catalyst can be obtained by replacing the corresponding precursor substances in proportion with reference to the above-mentioned examples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com