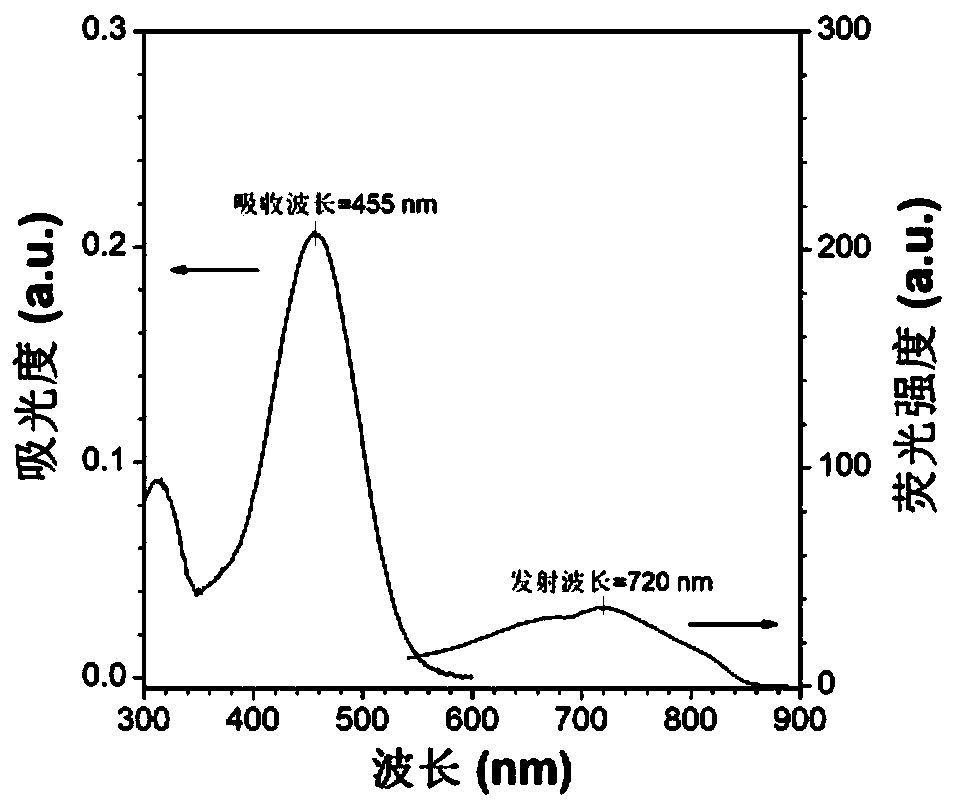
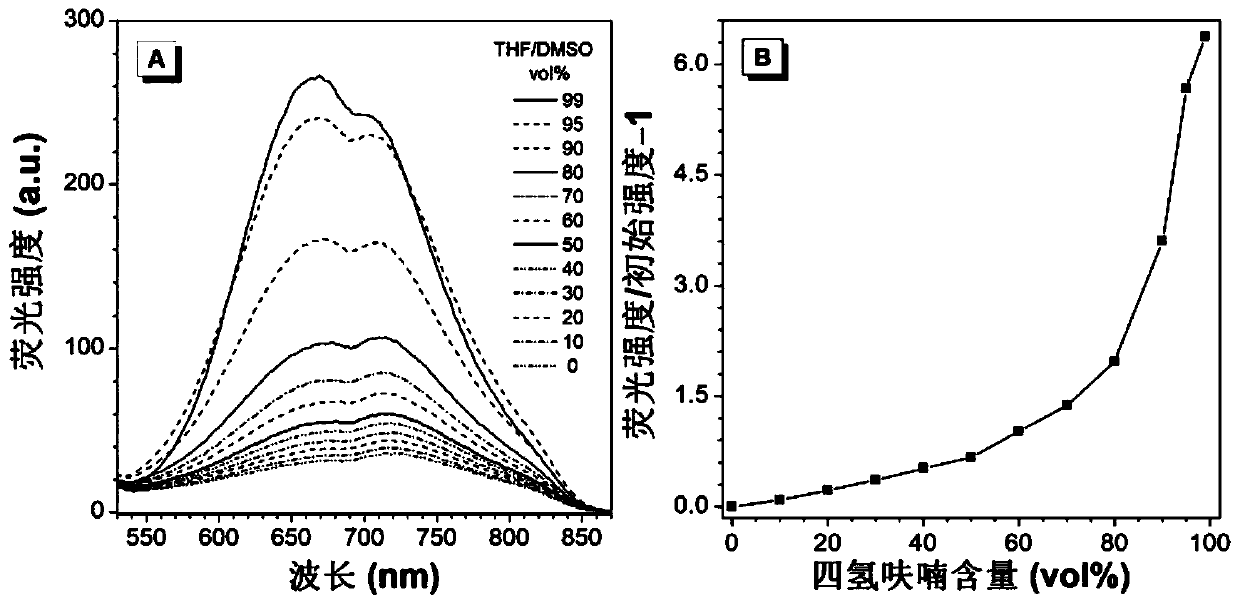
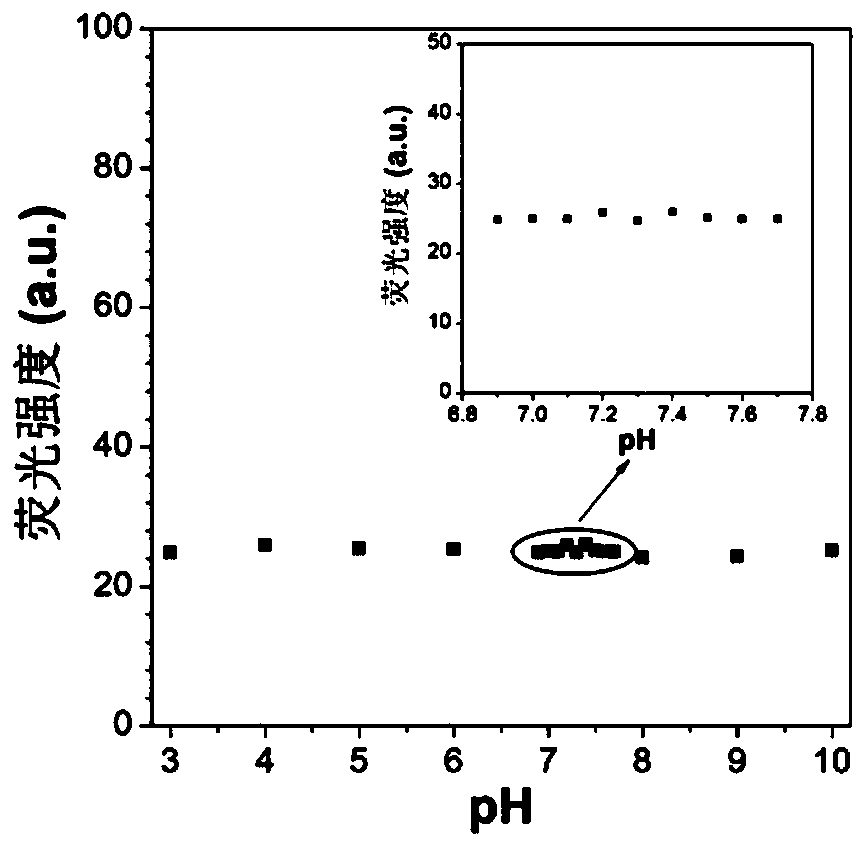
Red/near-infrared AIE probe and preparation method thereof, and application of red/near-infrared AIE probe in detection of Abeta aggregate and fibrotic plaques of Abeta aggregate
A near-infrared, aggregate technology, applied in the field of fluorescent probes, can solve the problems of the probe's limited ability to penetrate the blood-brain barrier, poor photostability, short probe excitation and emission wavelengths, etc., to achieve photostability and photostability. The effect of excellent bleaching, high signal-to-noise ratio and resolution, and best in vivo imaging performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (Z)-3-(4-(4-(1-cyano-2-(4-(dimethylamino)phenyl)vinyl)phenyl)pyridin-1-yl-1-yl)propane- 1-sulfonic acid sodium salt
[0045] Under nitrogen protection, add 1.1–1.5 equivalents of 4-pyridineboronic acid, 1 equivalent of 4-bromophenylacetonitrile and 0.01–0.1 equivalents of tetrakistriphenylphosphine palladium(0) to tetrahydrofuran / water mixture of potassium carbonate, 85–100 Stir and reflux at ℃ for 10-12 hours. After the reaction, add water, extract with dichloromethane three times, evaporate the solvent, perform silica gel column chromatography (petroleum ether: ethyl acetate), and obtain the product of the first step. Under the protection of nitrogen, the product of the first step and 2-5 equivalents of sodium 3-bromopropanesulfonate were refluxed in acetonitrile for 48-96 hours, filtered to obtain a solid, and then washed with acetonitrile and water respectively to obtain the product of the second step. Under the protection of nitrogen, the second step product and 0...
Embodiment 2
[0050] (Z)-3-(4-(4-(1-cyano-2-(4'-(dimethylamino)-[1,1'-biphenyl]-4-yl)vinyl)phenyl )pyridin-1-ium-1-yl)propane-1-sulfonic acid sodium salt
[0051] The raw materials are: bromine-substituted aryl acetonitrile is (1 equivalent of 4-bromophenylacetonitrile), arylboronic acid is (1.1-1.5 equivalents of 4-pyridineboronic acid), and linear halogenated compound is (2-5 equivalents of 3-bromopropane Sodium sulfonate) aldehyde-substituted aromatic compound is (0.9-1.2 equivalents of 4'-(dimethylamino)-[1,1'-biphenyl]-4-formaldehyde)
[0052] Preparation method is with embodiment 1, 1 H NMR (400MHz, DMSO-d 6 )δ (ppm): δ9.14 (d, J = 6.9Hz, 2H), 8.60 (d, J = 6.9Hz, 2H), 8.31–8.21 (m, 3H), 8.05 (dd, J = 13.3, 8.6 Hz, 4H), 7.85(d, J=8.5Hz, 2H), 7.69(d, J=8.9Hz, 2H), 6.84(d, J=8.9Hz, 2H), 4.74(t, J=6.7Hz, 2H), 2.98(s, 6H), 2.46(d, J=7.0Hz, 2H), 2.29–2.23(m, 2H).
[0053] The specific structure of the product obtained is as follows:
[0054]
Embodiment 3
[0056] (Z)-3-(4-(4-(1-cyano-2-(5-(4-(dimethylamino)phenyl)thiophen-2-yl)vinyl)phenyl)pyridine-1 -yl-1-yl)propane-1-sulfonic acid sodium salt
[0057] Bromine-substituted aryl acetonitrile is (1 equivalent of 4-bromophenylacetonitrile) aryl boronic acid is (1.1-1.5 equivalents of 4-pyridine boronic acid), and the linear halogenated compound is (2-5 equivalents of 3-bromopropanesulfonate sodium) Aldehyde-substituted aromatic compounds are (0.9-1.2 equivalents of 5-(4-(dimethylamino)phenyl)thiophene-2-carbaldehyde)
[0058] Preparation method is with embodiment 1, 1 H NMR (400MHz, DMSO-d 6 )δ (ppm): δ9.12 (d, J = 6.9Hz, 2H), 8.58 (d, J = 6.9Hz, 2H), 8.49 (s, 1H), 8.23 (d, J = 8.7Hz, 2H) ,7.95(d,J=8.6Hz,2H),7.77(d,J=4.2Hz,1H),7.61(d,J=8.9Hz,2H),7.51(t,J=5.6Hz,1H),6.80 (d, J=9.0Hz, 2H), 4.72(t, J=6.9Hz, 2H), 2.99(s, 6H), 2.45(d, J=7.0Hz, 3H), 2.29–2.23(m, 2H) .
[0059] The specific structure of the product obtained is as follows:
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com