Oral chimeric protein for inducing pancreas regeneration based on cholera toxin multimer structure and application of oral chimeric protein
A cholera toxin and chimeric protein technology, applied in the field of chimeric proteins, can solve the problems of apoptosis, inability to solve islet beta cells, side reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Construction of expression vector
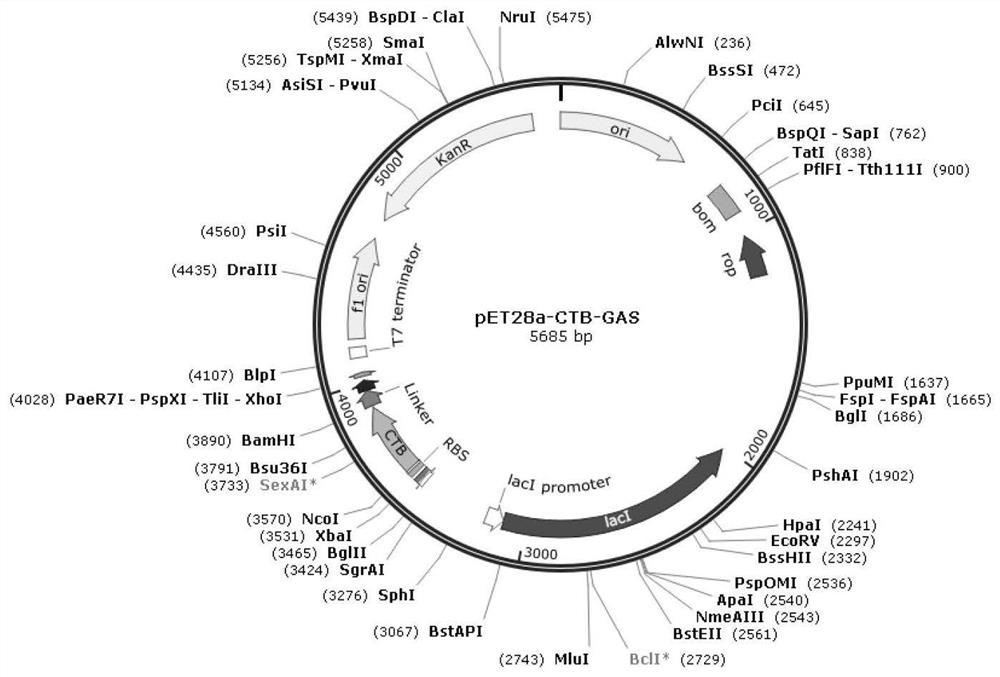
[0050] see figure 1 , is the construction diagram of pET28a-CTB-Linker-GAS plasmid in the embodiment of the present invention.
[0051] Connect the linker peptide (the end contains the furin cleavage site RRRR sequence) and the gastrin GAS sequence (the end contains the furin cleavage site RRRR sequence) sequentially and genetically synthesize to obtain Linker-GAS, and then proceed according to the conventional method Nde I and Xho I double enzyme digestion, and carry out Nde I and Xho I double enzyme digestion on the vector pET28a-CTB (purchased from addgene) at the same time; link the Linker-GAS fragment after digestion with pET28a-CTB after digestion, Obtain the recombinant plasmid pET28a-CTB-Linker-GAS of fusion expression, transform Escherichia coli BL21 (DE3), obtain the second polypeptide ((CTB-Linker-GAS) that can express 5 ) recombinant engineering bacteria pET28a-CTB-Linker-GAS / BL21.
[0052] The second polypept...
Embodiment 2
[0059] Example 2 Expression of fusion protein
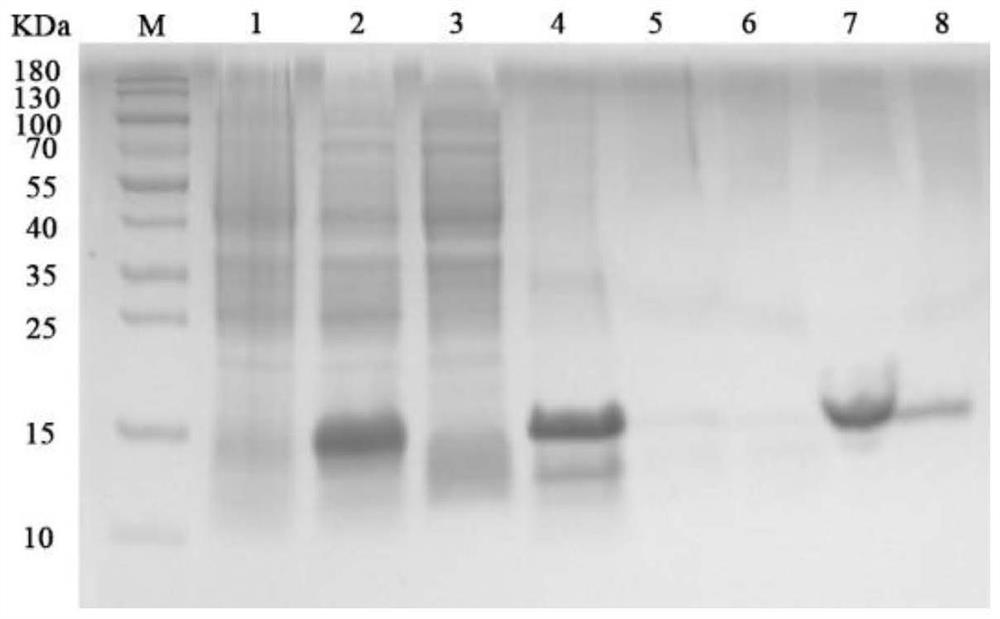
[0060] The pET28a-CTB-Linker-GAS / BL21 obtained in Example 1 was used for protein expression, and the inclusion bodies were obtained by cracking and freeze-thawing, and the protein was purified by denaturation and renaturation, and obtained by lyophilization (CTB-Linker-GAS) 5 Protein powder, the specific method is as follows:
[0061] (1) Protein expression: Add 7 μL ampicillin stock solution (50 mg / mL stock solution, 50 μg / mL final concentration) and 10 μL pET28a-CTB-Linker to 6 test tubes containing 7 mL LB medium on a clean bench. -GAS / BL21 glycerol tube bacteria solution, place the test tube in a constant temperature culture shaking box at 37°C and shake the bacteria at 200rpm for 12 hours overnight; after 12 hours, take out the 6 test tubes that have been cultured overnight, and press 2% on the ultra-clean workbench The (v / v) inoculum was transferred to conical flasks containing 350 μL ampicillin stock solution (50 mg / mL st...
Embodiment 3
[0066] Embodiment 3 fusion protein (CTB-Linker-GAS) 5 purification of
[0067] In this example, the dissolved supernatant prepared in Example 2 was purified by affinity chromatography after dialysis and renaturation, and obtained by lyophilization (CTB-Linker-GAS) 5 Protein powder, a total of 64 hours; Among them, the specific method of protein purification is as follows:
[0068] (1) Dialysis renaturation: cut the dialysis bag into small sections of 15-20 cm, in a large volume (300 ml) of 2% (W / V) NaHCO 3 Boil the dialysis bag with 1mol / L EDTA-2Na (pH=8.00) for 10min; after thoroughly washing the dialysis bag with distilled water, put the dissolving supernatant obtained in Example 2 and clamp both ends with clips, put into 500mL In the beaker, dialyze in the external liquid of 6, 4, 2, 1, 0.5 and 0mol / L urea solution (solvent is 25mmol / L Tris-HCl) successively, at 0mol / L, dialyze at least 3 times, and change it every 8h liquid (dialysis time can be adjusted in the early st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com