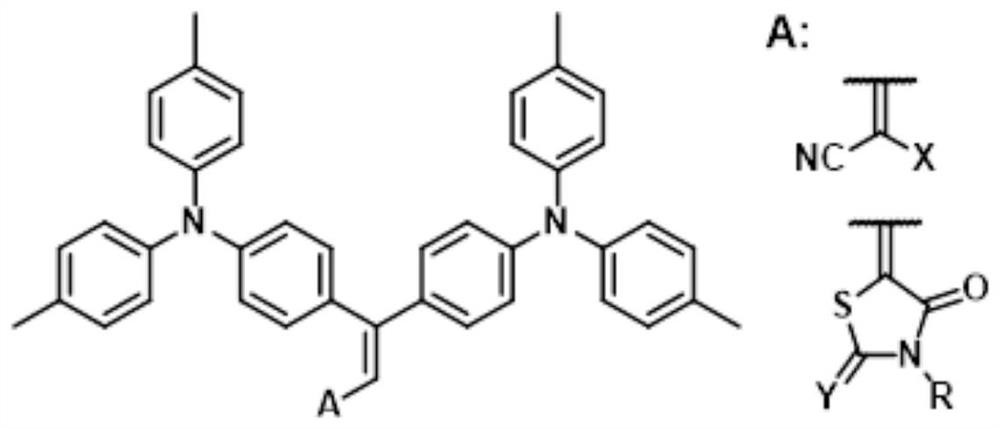
A class of merocyanine dyes with triphenylamine as donor, its preparation method and application
A technology of cyanine dyes and triphenylamine, which is applied to the preparation of styrene-based dyes, organic compounds, materials for organic semiconductor devices, etc., can solve the problems of poor stability and insufficient efficiency of perovskite solar cells, and achieve easy purification, high High energy conversion efficiency, stability, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] Regarding the preparation of unsaturated alkenal, in some embodiments, it may specifically be: adding phosphorus oxychloride dropwise to N,N-dimethylformamide in an ice-water bath under nitrogen or inert gas atmosphere. After stirring for 5-30 minutes, add 1,1-bis(4-N,N-di-p-R substituted phenylaminophenyl)ethylene in N,N-dimethylformamide solution dropwise to the above mixing in solution. After stirring for a period of time at 0-40°C (certainly also at room temperature), add an aqueous solution of sodium hydroxide to adjust the system to alkalinity, extract the reaction mixture with an organic solvent (such as dichloromethane), and dry (for example, by Add anhydrous sodium sulfate to realize), filter, and the crude product is purified by column chromatography to obtain the unsaturated alkenal shown in general formula (3).
[0072]
[0073] In some examples, the molar ratio of phosphorus oxychloride to N,N-dimethylformamide is 1:1-2.5.
[0074] In some examples, th...
Embodiment 1
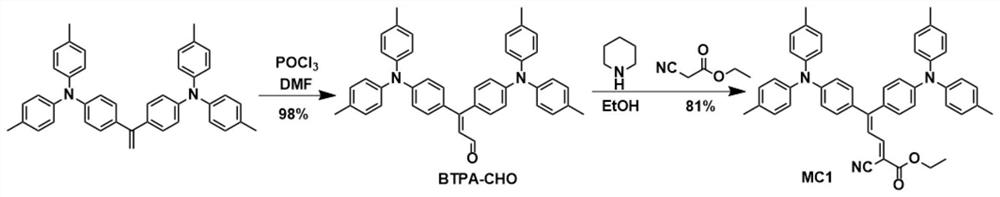
[0086] Synthesis of compound MC1: the synthetic route is as figure 2 shown.
[0087] Synthesis of Unsaturated Enaldehyde BTPA-CHO
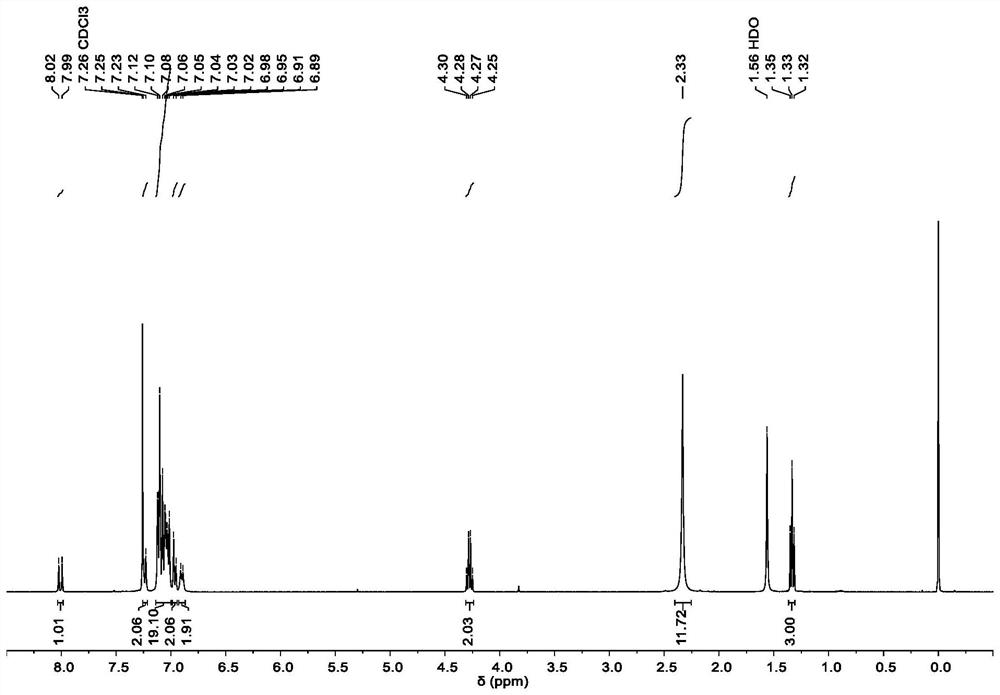
[0088] 0.14 mL of phosphorus oxychloride (1.5 mmol) was slowly added dropwise to 0.2 mL of N,N-dimethylformamide in an ice-water bath under a nitrogen atmosphere. After stirring for 15 minutes, a solution of 350 mg of 1,1-bis(4-(di-p-tolylamino)phenyl)ethylene (0.6 mmol) in 1 mL of N,N-dimethylformamide was added dropwise to the above mixed solution middle. After stirring for 5 hours at 25°C, add an aqueous solution of sodium hydroxide to adjust the system to alkalinity, extract the reaction mixture with dichloromethane, dry with anhydrous sodium sulfate, filter, and separate through a silica gel column after removing the solvent (eluent dichloromethane), the crude product was purified by column chromatography to obtain 361 mg of yellow solid BTPA-CHO with a yield of 98%. 1 H NMR (400MHz, Chloroform-d) δ9.54(d, J=8.0Hz, 1H), 7.22(d, J=8.0Hz, ...
Embodiment 2
[0092] Synthesis of compound MC2
[0093] Under nitrogen, after dissolving 180mg of unsaturated enaldehyde BTPA-CHO (0.30mmol) and 85mg of 3-ethylrhodanine (0.15mmol) in 4mL of dry tetrahydrofuran, a catalytic amount of piperin was added to the mixture Pyridine. The mixed system was heated to reflux, stirred overnight, and then separated by extraction with water and dichloromethane. The obtained organic phase is dried with anhydrous sodium sulfate, filtered, and after removing the solvent, carry out silica gel column chromatography separation (eluent is dichloromethane: sherwood oil, volume ratio is 1: 1), product ethyl acetate After recrystallization and drying, 152 mg of the red compound MC2 was obtained with a yield of 68%. 1 H NMR (600MHz, Chloroform-d) δ7.49 (d, J=12.0Hz, 1H), 7.20-7.17(m, 2H), 7.13-7.02(m, 18H), 6.99-6.96(m, 2H), 6.93-6.89(m,2H),6.43(d,J=12.0Hz,1H),4.14(q,J=7.2Hz,2H),2.33(s,6H),2.33(s,6H),1.25(t ,J=7.2Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com