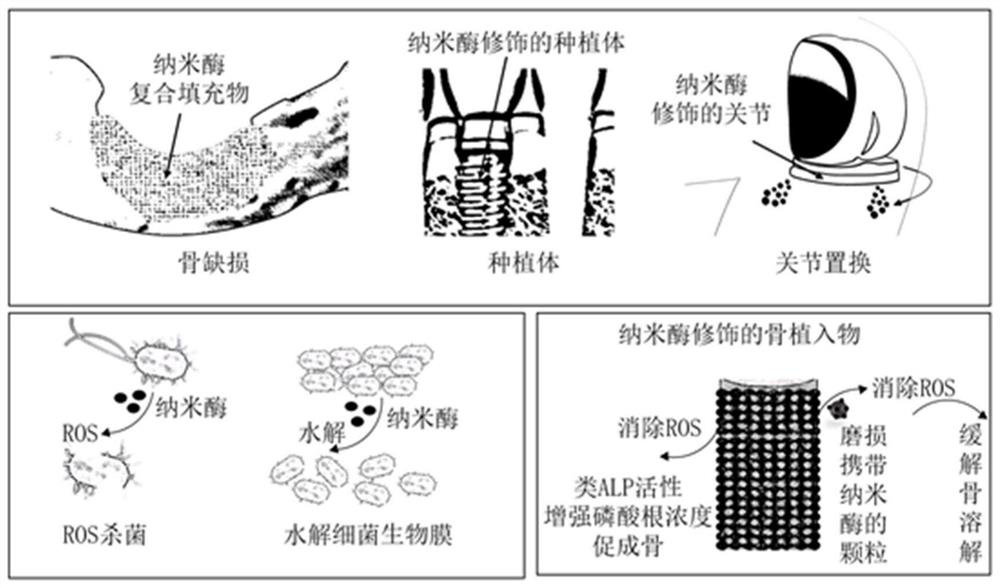
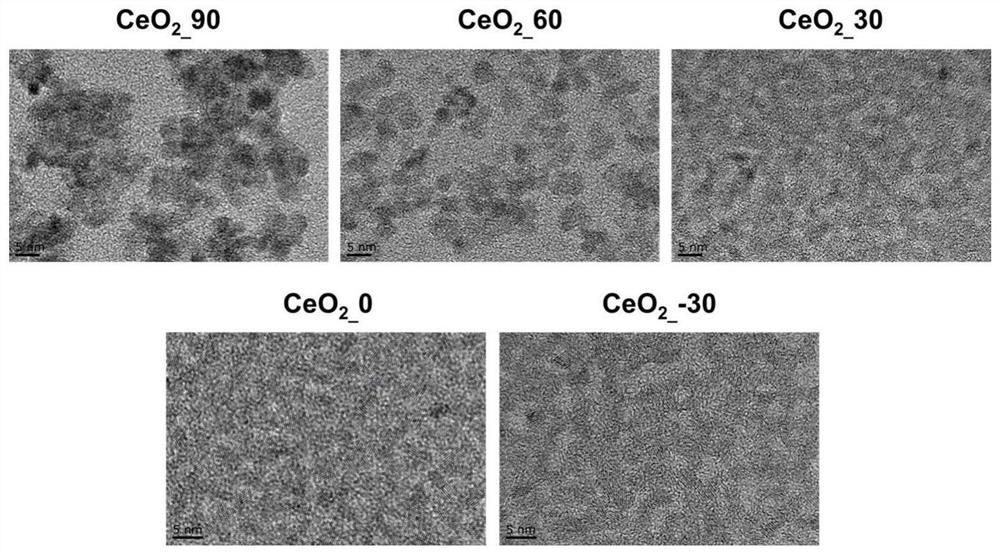
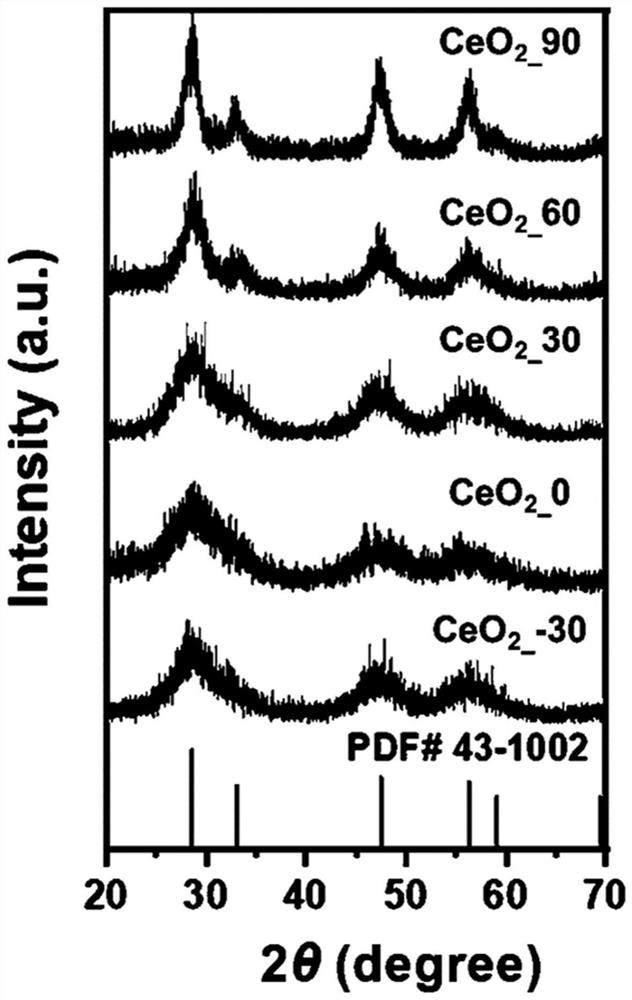
Bone implant material based on nano-enzyme drug modification and preparation method and application thereof
A technology of bone implant material and modification method, applied in the field of bone implant material and its preparation, can solve the problems of enhancing local inflammatory ROS, without actively promoting osseointegration, osteolysis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1 Preparation of Bone Implant Material Loaded with Cerium Oxide Nanozyme by Alkali Activation Pretreatment on Titanium Surface
[0098] Titanium surface alkali-activated pretreatment bone implant material loaded with cerium oxide nanozyme was prepared according to the following method:
[0099] (1) Titanium surface alkali activation preconditioning:
[0100] ① Take pure titanium sheet or titanium wire, use absolute ethanol, acetone and pure water to ultrasonically clean 3 times, each time for 10 minutes, and dry it for later use;
[0101] ② Treat the titanium sheet or wire in ① with 3 moles per liter of sodium hydroxide solution at 80 degrees Celsius for 12 hours, then use absolute ethanol and water to soak and ultrasonically clean it 3 times, each time for 10 minutes, and dry it for later use;
[0102] (2) Surface modification of cerium oxide nanozyme to pretreated titanium sheet or titanium wire in (1):
Embodiment 2
[0114] Example 2 Preparation of bone implant material loaded with zirconium-doped cerium oxide nanozyme by pretreatment of titanium oxide nanotubes on titanium surface
[0115] Titanium surface titanium oxide nanotube pretreatment Bone implant material loaded with zirconium-doped cerium oxide nanozyme was prepared according to the following method:
[0116] (1) Pretreatment of titanium oxide nanotube arrays on titanium surface:
[0117] ① Take pure titanium sheet or titanium wire, use absolute ethanol, acetone and pure water to ultrasonically clean 3 times, each time for 10 minutes, and dry it for later use;
[0118] ② Preparation of anodic oxidation electrolyte: 0.9128g NH 4 F, 4.5mL H 3 PO 4 (95-98%), 62.5mL water and 62.5mL ethylene glycol, spare;
[0119] ③Anodizing treatment:
[0120] i Reaction parameters: Use the titanium sheet or titanium wire in ①, put the anodic oxidation electrolyte prepared in ② in a 100mL plastic beaker, and use a constant voltage (30V) DC sy...
Embodiment 3
[0132] Example 3 Preparation of high molecular weight polyethylene bone implant material doped with cerium oxide nanozyme in situ
[0133] In situ cerium oxide nanozyme-doped high molecular weight polyethylene bone implant material was prepared according to the following method:
[0134] (1) Synthesis and preparation of cerium oxide in-situ loaded polyethylene particles:
[0135] ① Dissolve 190.55 mg of cerium acetylacetonate in 15 mL of absolute ethanol as the precursor of cerium oxide nanozyme, and set aside;
[0136] ② Take 1,500 mg of polyethylene with a molecular weight of 2 million and place it in 100 mL of xylene, stir at 110°C for 6 hours to obtain a polyethylene solution, and set aside;
[0137] ③The precursor solution obtained in ① was slowly added dropwise to the ② system by using a syringe pump, and stirred vigorously for 24 hours to prepare a polyethylene material doped and fixed in situ with cerium oxide;
[0138] ④ Slowly add the solution obtained in ③ into 60...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com