Diamine compound, resin, photosensitive resin composition, and cured film
A technology of amine compounds and photosensitive resins, which is applied in the field of diamine compounds, resins, photosensitive resin compositions and cured films, can solve the problems of high thermal expansion coefficient of photosensitive resin cured films, reduced exposure sensitivity, and influence on transmittance, etc., to achieve Good photosensitive properties, excellent mechanical properties, and the effect of improving light transmittance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
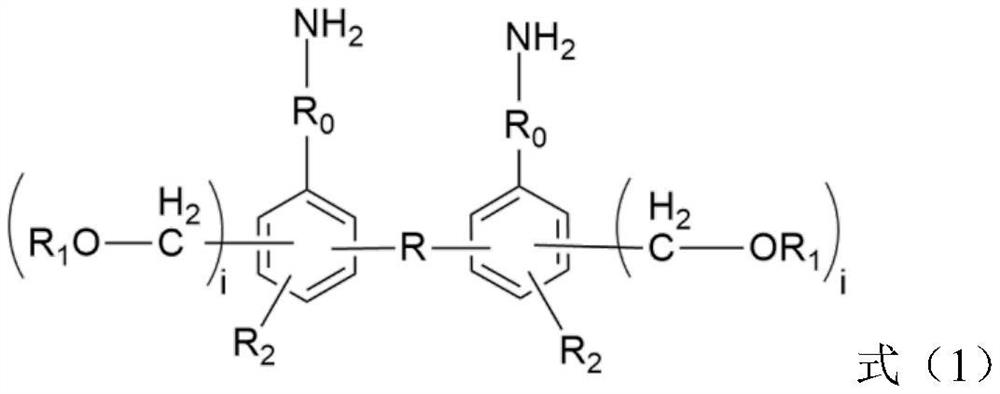
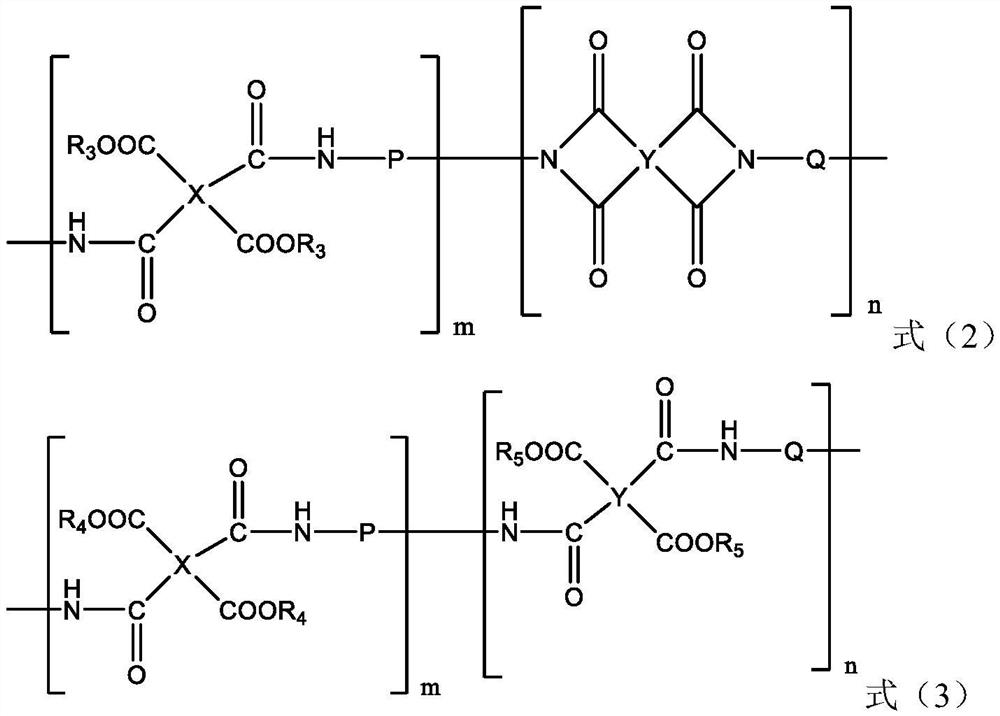
[0037] for R 0 Indicates -Ph-SO 3 - the diamine compound, R 1 Indicates -CH 3The preparation method comprises the steps of: mixing the phenolic compound shown in formula (4) and p-nitrobenzenesulfonyl chloride in a solvent such as DMF according to a molar ratio of 1:2, adding potassium carbonate, and reacting to obtain a dinitro compound; the obtained The dinitro compound is reduced under the Pd-C catalyst / hydrogen atmosphere / ethylene glycol monomethyl ether solvent to obtain the diamine compound. The general reaction formula is shown in the following formula (5). for R 1 The formula (4) representing a hydrogen atom needs to first esterify the alcoholic hydroxyl group in the formula (4) to protect the hydroxyl group, then react with p-nitrobenzenesulfonyl chloride, and then restore the alcoholic hydroxyl group through the step of hydrolysis of the ester group.
[0038]
[0039] Furthermore, for R 0 A diamine compound representing -Ph-S-, wherein -Ph represents a pheny...
Embodiment
[0089] The above and other advantages of the present invention can be better understood through the following examples, but the following examples are not intended to limit the scope of the present invention.
[0090] Description of abbreviations:
[0091] ODA: 4,4'-diaminodiphenyl ether
[0092] 6FAP: 2,2-bis(3-amino-4-hydroxyphenyl)hexafluoropropane
[0093] SiDA: 1,3-bis(3-aminopropyl)-1,1,3,3-tetramethyldisiloxane
[0094] 6FDA: 2,2'-bis(3,4-dicarboxylic acid phenyl)hexafluoropropane dianhydride
[0095] ODPA: 3,3’,4,4’-Diphenyl ether tetracarboxylic dianhydride
Synthetic example 1
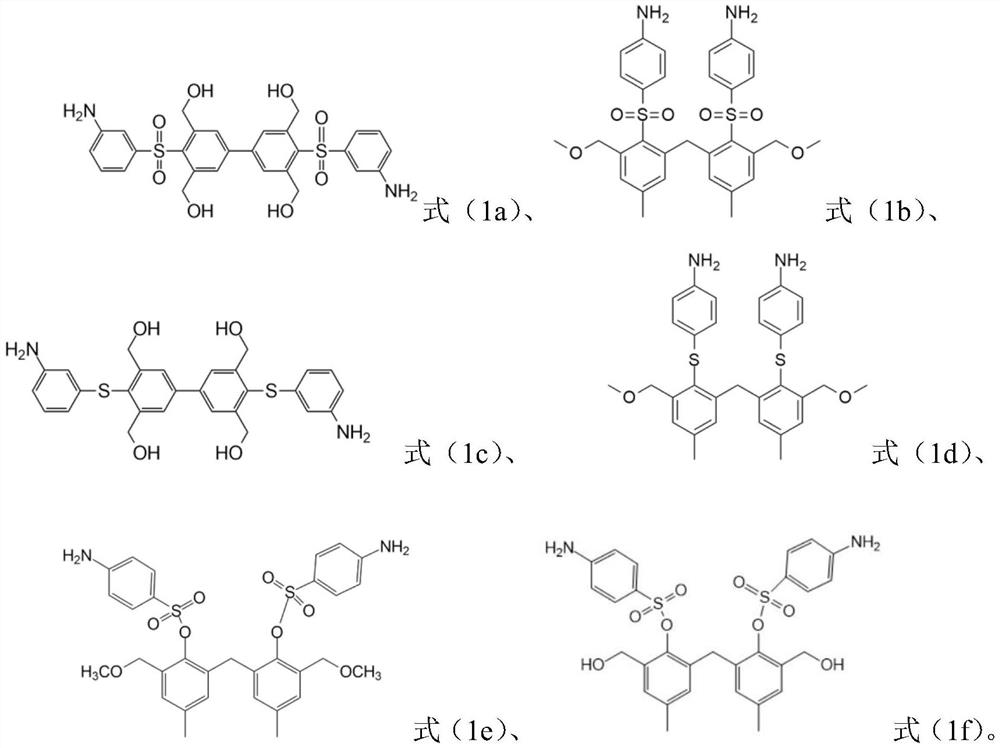
[0097] Synthesize diamine compound Y1 according to the following route:
[0098]
[0099] The specific operation is: use 2,6-dimethylbromobenzene as the raw material, oxidize the methyl group to a carboxyl group, replace the benzene ring with a sulfonic acid group, and convert the sulfonic acid group into a sulfonyl chloride through phosphorus oxychloride After being reduced to thiophenol, react with m-nitrobromobenzene to generate thioether compounds, and then in bis(pinacolate) diboron (B 2 Pin 2 ) under the action of a Suzuki coupling reaction to obtain a thioether biphenyl compound, and finally to obtain a diamine compound Y1 through hydrolysis and reduction.
[0100] Or select the following route to synthesize the diamine compound Y1 derivative:
[0101]
[0102] The specific operation is: use 3,3',5,5'-tetraaldehyde-4,4'-dihydroxybiphenyl as the raw material, and react with phosphorus tribromide to obtain bromide, and then in the mixed solvent of quinoline and pyri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com