Method for functionalizing zirconium oxide toughened aluminum oxide surface by using silicon nitride
A surface functional, silicon nitride technology, applied in pharmaceutical formulations, prostheses, metal processing equipment, etc., can solve problems such as osteoblast apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0047] Example 1: Surface Functionalization Procedure
[0048] ZTA samples ( CeramTec, GmbH, Plochingen, Germany) was machined from a femoral head with a diameter of 36 mm (production year: 2014). Samples (10 x 10 mm with a thickness of 3 mm) were cut using a low speed diamond-coated blade and finely polished to a roughness of about ten to twenty nanometers.
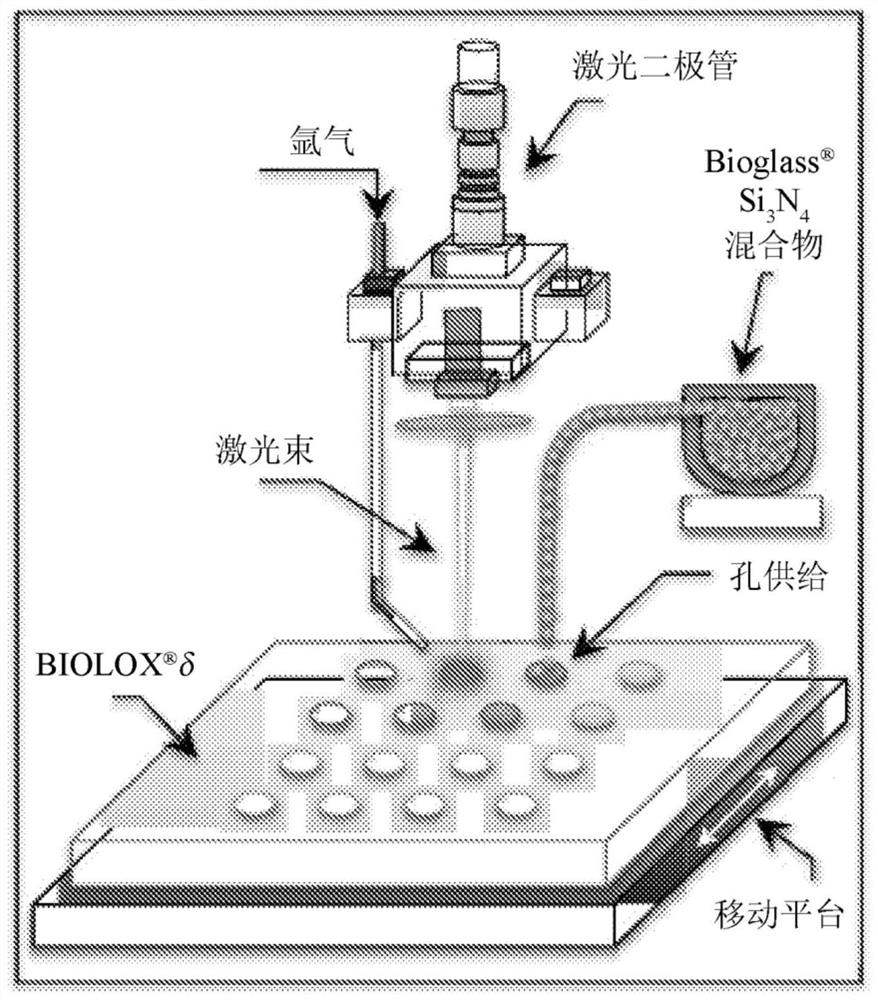
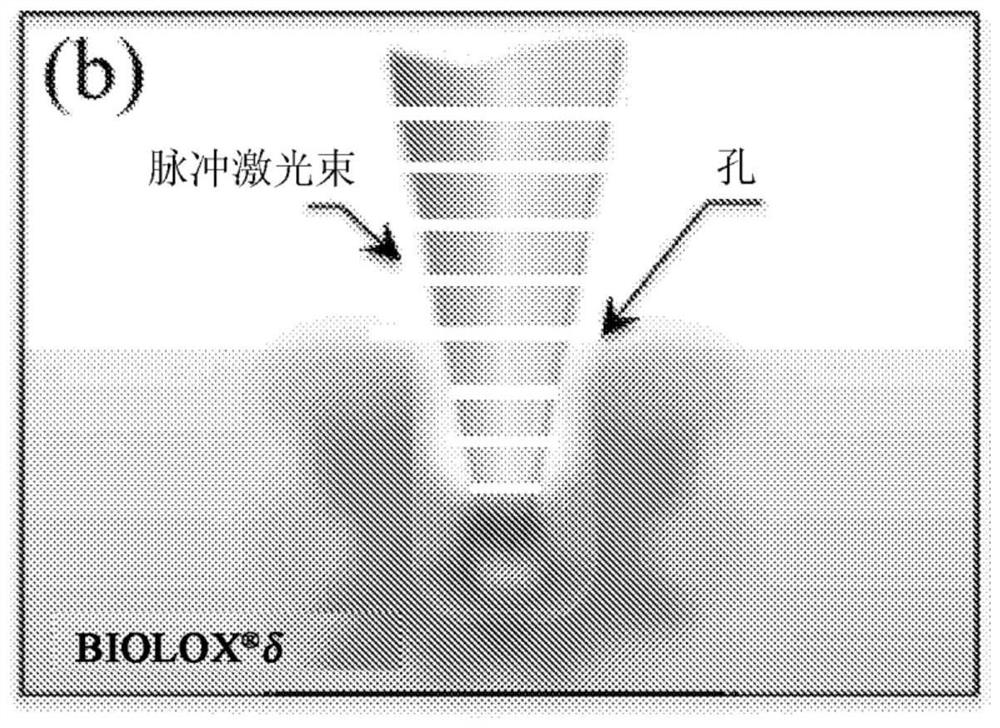
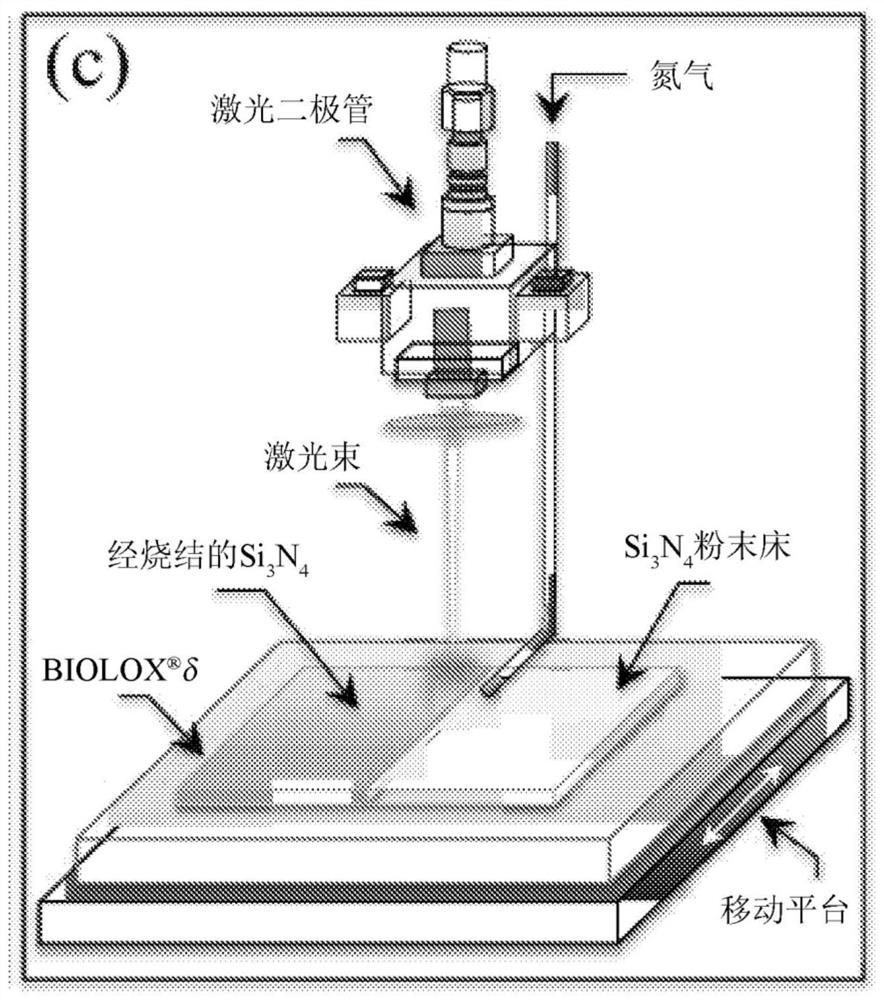
[0049] Laser patterning procedure
[0050] The device used for laser patterning of the ZTA surface was a VisionLWI V ERGO-workstation equipped with a Nd:YAG laser (wavelength 1064 nm). The focus distance, nominal maximum power and burst energy were set to 250mm, 17kW and 70J, respectively, while the applied potential and discharge time were adjusted in the range of 160-500V and 1-20 milliseconds, respectively. The laser patterning workstation was equipped with a gas nozzle connected to an argon source at 1.2 atm to locally limit the presence of oxygen at the location of the laser impingement. A motorized x-y stage...
example 2
[0054] Example 2: Surface Characterization Method
[0055] Characterization was performed with a confocal scanning laser microscope capable of obtaining high-resolution optical images with depth selectivity (Laser Microscopy 3D and Profilometry, Keyence, VKx200 series, Osaka, Japan). Surface morphology of the textured ZTA surface before and after powder mixture filling. All images were collected using 20x magnification. Si was obtained using scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) (JSM-700 1F, Japan Electron Optics Laboratory, Tokyo, Japan (JEOL, Tokyo, Japan)). 3 N 4 High-resolution images and chemical composition maps of the coated substrates.
[0056] FTIR spectra were obtained using a high sensitivity spectrometer (Spectrum 100FT-IR Spotlight400, Perkin-Elmer Inc., Waltham, MA, USA). The spectral resolution of this device is 0.4cm -1 . The average FTIR spectrum for each substrate was calculated from eight independent measure...
example 3
[0057] Example 3: Cell Culture and Bioassays
[0058] SaOS-2 human osteosarcoma cells were incubated in 4.5g / L glucose DMEM supplemented with 10% fetal bovine serum (D-glucose, L-glutamine, phenol red and sodium pyruvate; Half Well Co., Ltd. (Nacalai Tesque, Kyoto, Japan). Kyoto, Japan)) for cultivation and incubation. Cells were then propagated in petri dishes at 37°C for 24 hours. After adjusting the final cell concentration to 5 × 10 5 After cells / ml, the cultured cells were deposited on Si that had been previously sterilized by exposure to UV light 3 N 4 On the surface of coated and uncoated ZTA substrates (n=3 for each type). By seeding the cells in osteogenic medium (DMEM supplemented with 50 μg / mL ascorbic acid, 10 mM β-glycerophosphate, 100 mM hydrocortisone, and about 10% fetal bovine calf serum), and then warming the samples at 37° C. After 7 days of incubation, bone conduction tests were performed. During one week of incubation, the medium was changed twice. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com