Preparation method of avibactam sodium
A technology of avibactam sodium and compound, applied in the field of drug synthesis, can solve the problems of affecting product quality, low yield, difficult to remove, etc., and achieve the effects of improving quality, high yield and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of compound 2:
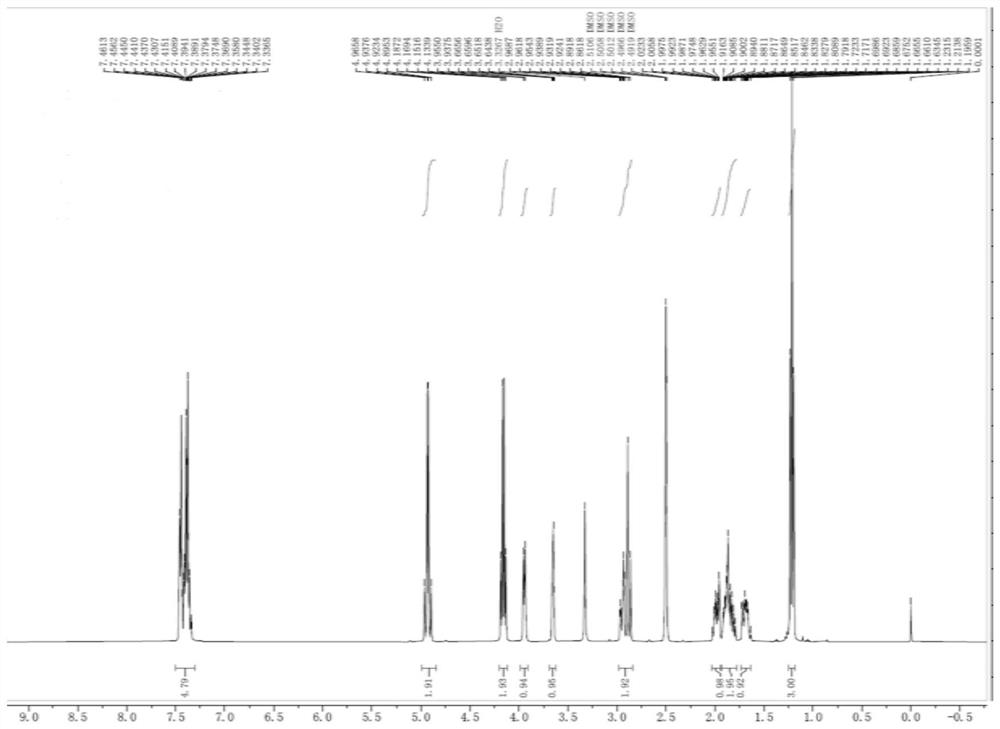
[0037] At room temperature, compound 1 (36.8 g, 0.1 mol) was put into 100 mL of dichloromethane, then 150 mL of 10% potassium carbonate solution was added, and after stirring for 0.5 h, the organic phase was separated. Then the organic phase was cooled to 0~5°C, DIPEA (19.4g, 0.15mol) was added, and a dichloromethane solution (150mL) of triphosgene (14.8g, 0.05mol) was slowly added dropwise. React at 5°C. When the reaction is complete, wash with 100 mL of 1M hydrochloric acid, 100 mL of saturated sodium bicarbonate solution and 100 mL of saturated sodium chloride solution, dry over anhydrous sodium sulfate, and filter. The solvent was then distilled off at 40°C. After distillation, 150 mL of methyl tert-butyl ether was added, stirred for 1 h, filtered and dried to obtain compound 2 (27.8 g, 0.0912 mol, 91.2%). HPLC%=99.2%, 1 H NMR (DMSO-d 6 , 400MHz)δ7.3365-7.4613(m, 5H), 4.8953-439658(m, 2H), 4.1339-4.1872(m, 2H), 3.9375-3.9550(d, 1H, ...
Embodiment 2
[0039] Preparation of compound 3
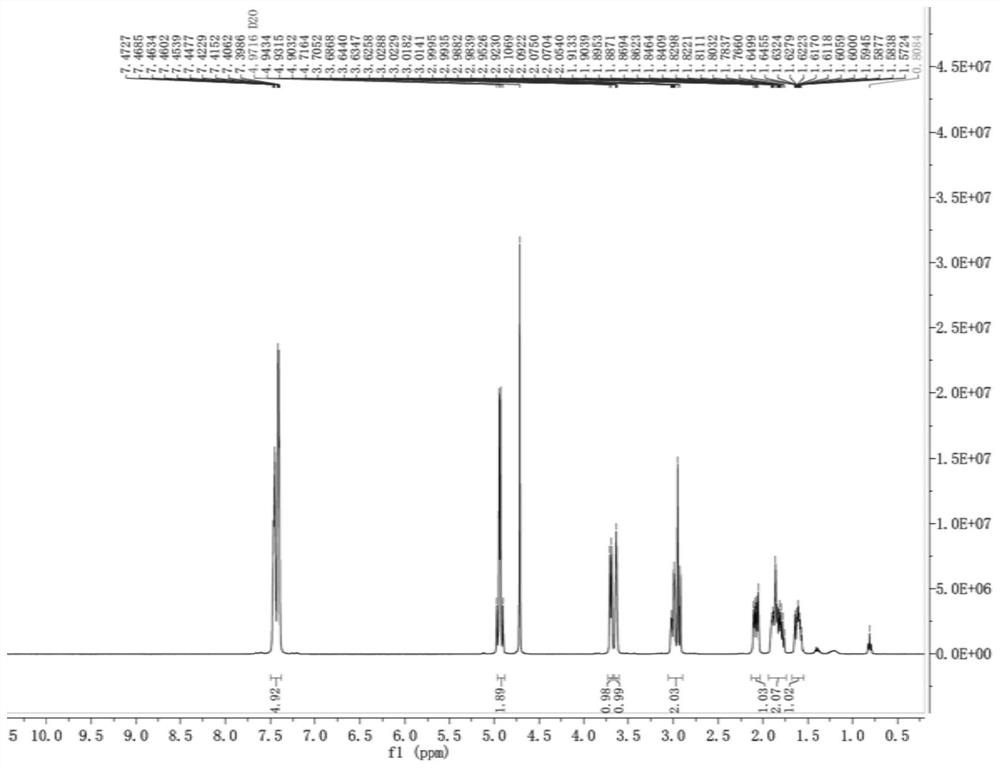
[0040] At room temperature, compound 2 (27.4 g, 0.09 mol) was dissolved in 150 mL of water and 150 mL of tetrahydrofuran, and then, under stirring, 32 mL of 10% lithium hydroxide solution was slowly added dropwise. Add 100 mL of ethyl acetate to the reaction solution, stir and separate the liquid to take the water phase, then use 1M hydrochloric acid to adjust pH=1~2, then add 200 mL of ethyl acetate to extract, add 24 g of 20% sodium carbonate solution to the reaction solution with stirring, and then stir Crystallize for 2 h, filter and dry to obtain compound 3 (24.6 g, 0.0824 mol, 91.6%). HPLC%=99.7%, 1 H NMR (DMSO-d 6 , 400MHz) δ7.3986-7.4727 (m, 5H), 4.9032-4.9434 (m, 2H), 3.6258-3.7052 (m, 2H), 2.9230-3.0288 (m, 2H), 1.5724-2.1069 (m, 4H). ESI-MS[M-Na] - m / z 275.
Embodiment 3
[0042] Preparation of compound 4
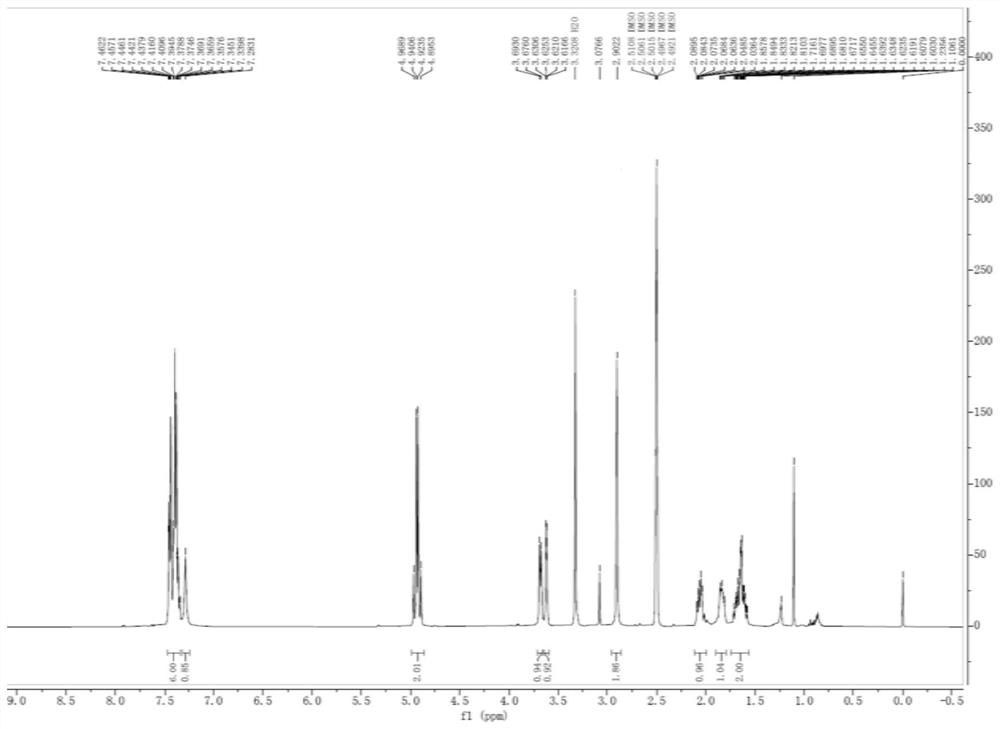
[0043] At room temperature, compound 3 (23.9 g, 0.08 mmol) was dissolved in 200 mL of dichloromethane, and then isobutyl chloroformate (10.9 g, 0.08 mmol) was slowly added, and after stirring for 0.5 h, the temperature was lowered to 0-5 °C, and then 20 mL of 25% ammonia water was slowly added dropwise, and after the dropwise addition was completed, the reaction was carried out for 1 h. After the reaction is completed, wash with 100 mL of saturated sodium bicarbonate solution and 100 mL of saturated sodium chloride solution, dry with anhydrous sodium sulfate, and filter. After distillation, 100 mL of methyl tert-butyl ether was added for crystallization for 2 h, and the mixture was filtered and dried to obtain compound 4 (19.5 g, 0.071 mol, 88.7%). HPLC%=99.8%, 1 H NMR (DMSO-d 6 , 400MHz)δ7.2831-7.4622(m, 7H), 4.8953-4.9689(m, 2H), 3.6760-3.6930(d, 1H, J=6.8Hz), 3.6166-3.6306(m, 1H), 2.9022(s, 2H), 1.6030-2.0895 (m, 4H). ESI-MS[M+1] + m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com