Use of bacteriophage outer membrane breaching proteins expressed in plants for the control of gram-negative bacteria
a technology of outer membrane and bacteriophage, which is applied in the direction of peptides, cyclic peptide ingredients, dna/rna fragmentation, etc., can solve the problems of compromising the “barrier function” of the gram negative outer membrane, bomb proteins compromising the integrity of the bacterial lps barrier, and not the inner membrane, so as to enhance the resistance of the plant to infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Use of a Plant Pathogen to Isolate Bacteriophage Capable of Infecting a Gram Negative Plant Pathogen, Xanthomonas pelargonii
[0205]An overnight culture of X. campestris pv. pelargonii (syn. X. pelargonii) strain CHSC was grown at 30° C. in PYGM medium (peptone, yeast extract, glycerol and morpholinepropanesulfonic acid; DeFeyter et al. 1990) with moderate shaking. Five ml of this overnight culture plus 50 ml of unsterilized water taken from the edge of a large pond in an agricultural setting was added to 50 ml of PYGM plus 2.5 g CaCO3 and allowed to incubate at 30° C. for 48 hours without shaking. Following incubation, 1 ml of this enrichment culture was centrifuged for 1 minute at 5000 g to remove most bacteria and debris, and 500 μl of the supernatant was removed and sterilized with a drop of chloroform. Droplets of this supernatant were placed atop an overlay plate containing strain CHSC in top agar. Overlay plates were PYGM agar plates overlayed with 200 μl of overnight CHSC bro...
example 2
Use of Agar Plate Overlay Assays to Characterize Phase Host Range and to Identify Phage with an Ability to Kill Bacterial Hosts that they Cannot Infect
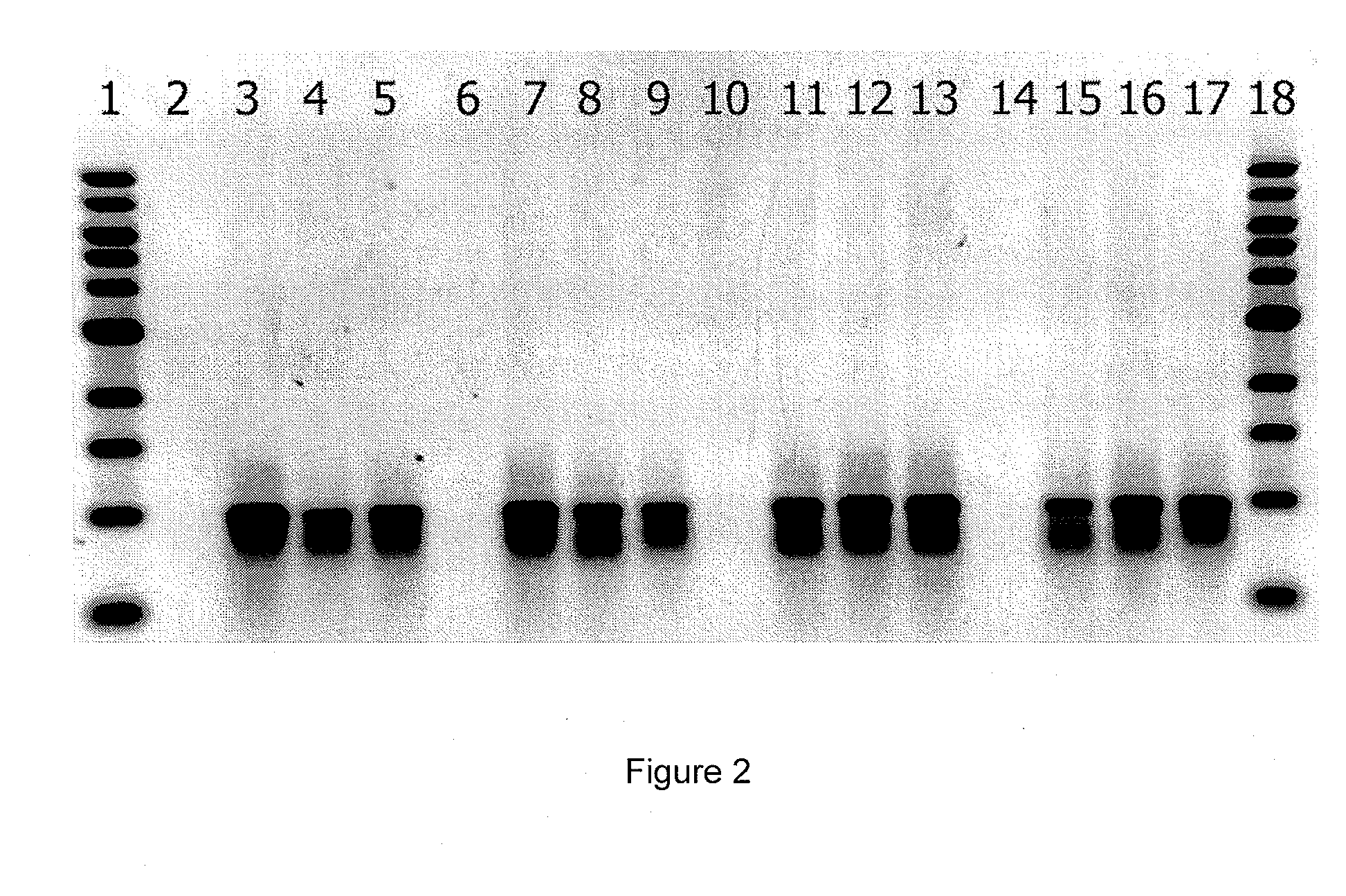
[0206]PYGM plates were overlaid with X. pelargonii CHSC and droplets of various purified phage samples obtained from Example 1 were added to the plates and incubated at 30° C. for 48 hours. All phage were able to infect CHSC and cause clear zones of lysis. Cell suspensions of overnight broth cultures of X. citri B21.2, X. campestris 528 and R. solanacearum G2 were added to 0.7% water agar as described in Example 1 and individually overlayed on the phage infected CHSC plates.
[0207]Plates were incubated an additional 48 hrs at 30° C. and phage were evaluated for ability to kill Gram negative bacteria that they could not infect from the outside. Some phage exhibited presence of a strong, apparently diffusible killing factor for all bacteria tested. Phage isolate 15 (P15) was selected for sequencing and further evaluation.
example 3
Use of Genomic Sequencing and Annotation Techniques to Identify Gene Candidates from Phage P15 Encoding Proteins with Ability to Kill Bacteria from the Outside
[0208]The P15 genome was completely sequenced in order to identify the gene(s) expressing the diffusible killing factor. P15 DNA was made according to standard protocols using X. pelargonii strain CHSC as the host bacterium. The P15 DNA was digested with EcoRV, yielding eleven fragments, ranging in size from 12.4 kb to 357 bp. Most of the fragments were cloned; some were not cloned, despite repeated attempts, most likely due to the presence of restriction endonucleases and holins. The cloned DNA fragments were used directly for sequencing, using vector-based primers initially, and primer walking thereafter until each fragment was completed. Fragments that were not cloned were sequenced using P15 genomic DNA. Fragment assembly was accomplished using P15 genomic DNA and primers extending outside each fragment in both directions....
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com