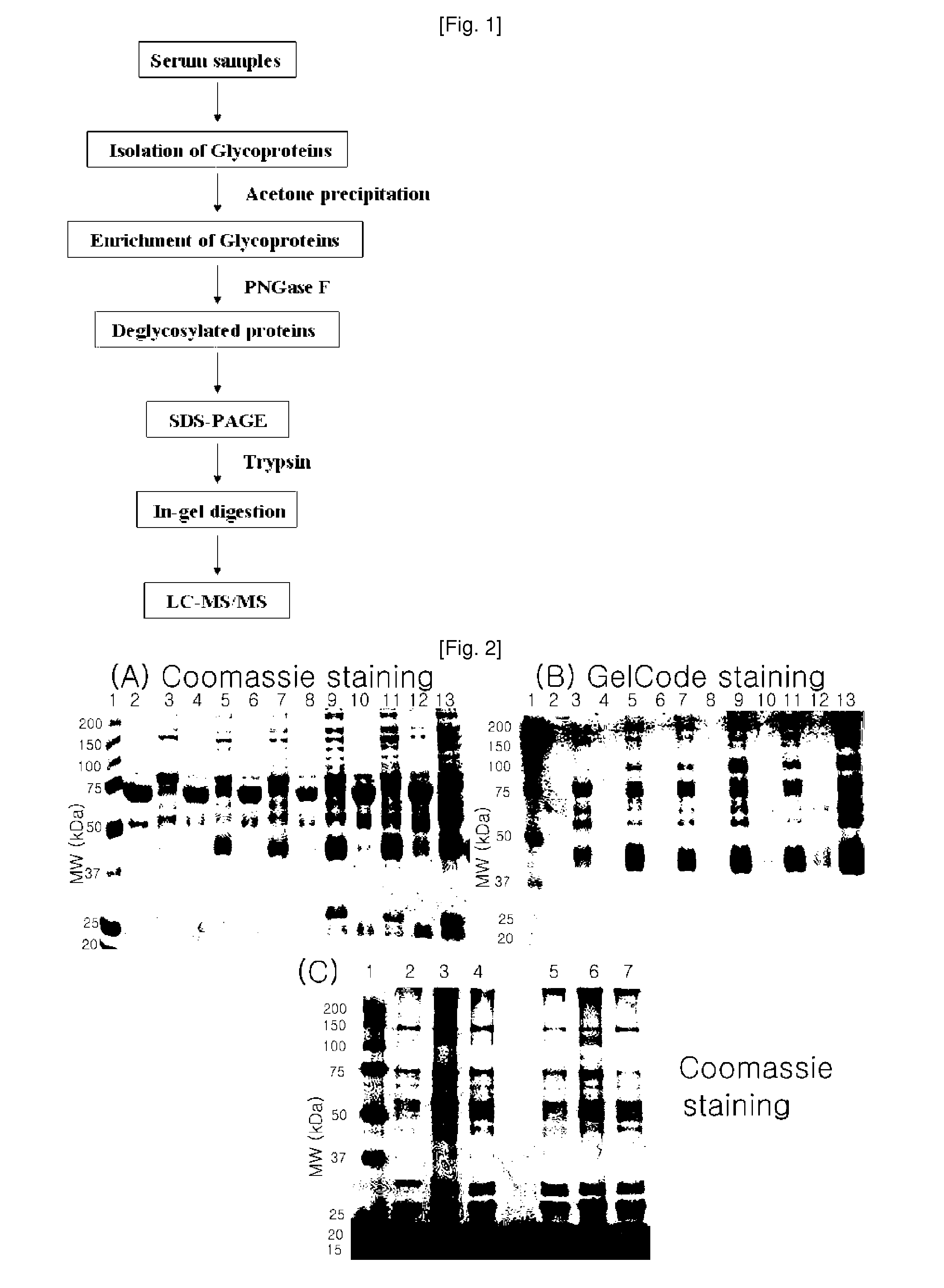
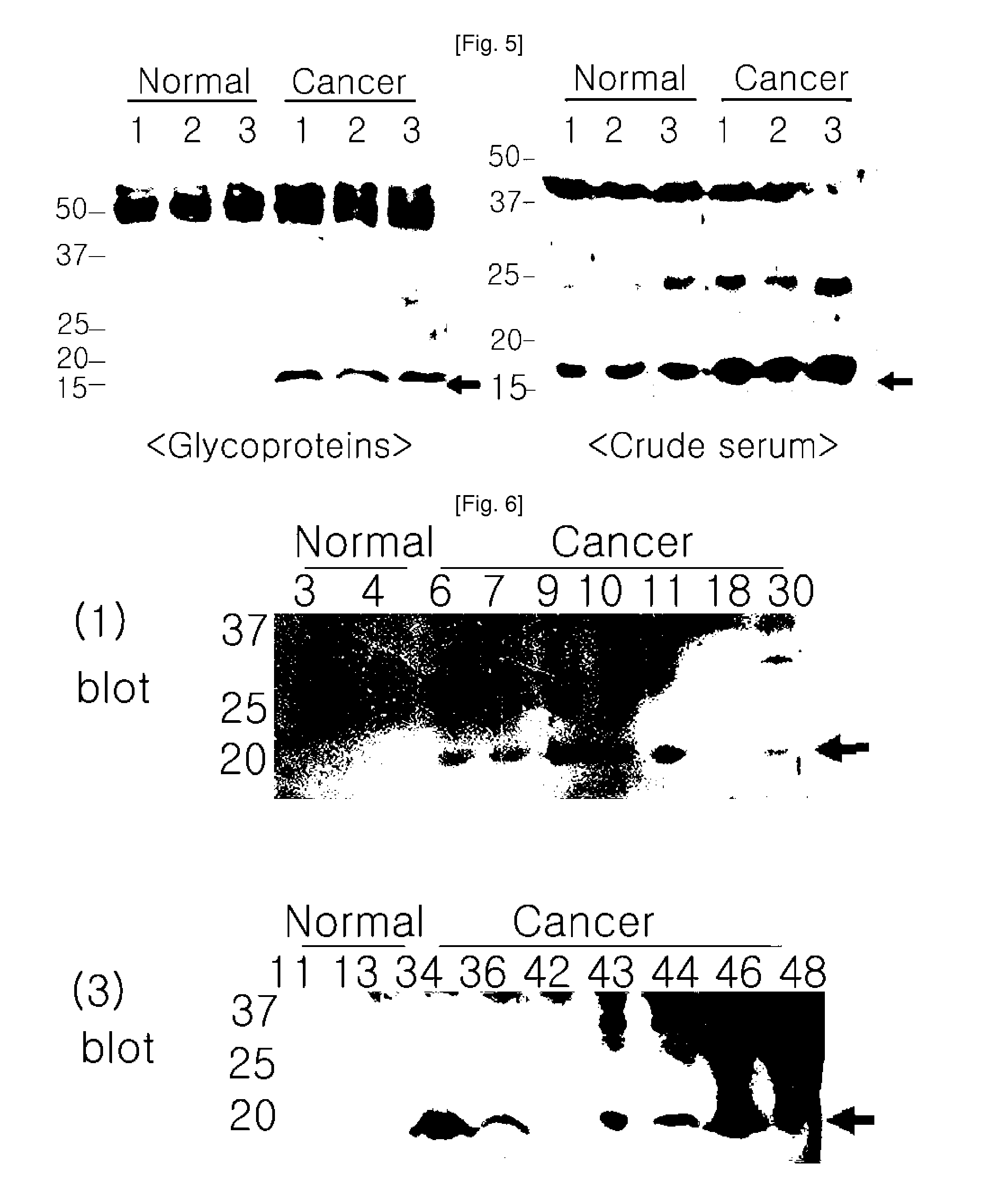
Plasma kallikrein fragments as diagnostic biomarkers for lung cancers
a technology of lung cancer and plasma kallikrein, which is applied in the field of plasma kallikrein fragment as a diagnostic biomarker for lung cancer, can solve the problems of difficult detection of low-abonding proteins which can be used as new biomarkers, increase in the incidence of lung cancer and the resulting mortality rate in korea, and achieve the effect of being useful in diagnosing lung cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation and Deglycosylation of Glycoprotein from Human Serum
[0080] Serum Sample
[0081]6 blood samples were collected from 3 lung adenocarcinoma patients and 3 healthy normal persons among 65-year-old smoking men under consent in Kyungpook National University Hospital, Korea (Table 1). The blood samples were centrifuged at 4° C. at 12000 rpm for 10 minutes to collect sera, which were then stored at −70° C. before use in experiments.
TABLE 1Features of lung cancer patients and normal patientsLung cancer groupNormal groupNumber of subjects 3 3Clinical historyLung cancerHealthySexMenMenAge6464SmokingSmokersSmokers
[0082] Isolation and Concentration of Glycoproteins Using Multi-Lectin Affinity Column
[0083]A multi-lectin affinity column was used to isolate and concentrate glycoproteins from the serum samples obtained in Example . The multi-lectin affinity column (Qiagen, USA) was packed with ConA (concanavalinA), LCH (lentil lectin), GNA (snowdrop lectin), WGA (wheat germ agglutinin), SNA ...
example 2
Deglycosylation of Glycoproteins and Preparation of Peptides
[0090] Deglycosylation of Glycoproteins
[0091]The serum glycoproteins concentrated in Example 1 were treated with peptide-N-glycosidase F (PNGase F) to remove the sugar moieties and were subjected to 1D-SDS PAGE.
[0092]15 □ of the concentrated glycoproteins were denatured in a buffer, containing 100 mM mercaptoethanol and 2% octyl beta-D-glucopyranoside, at 100° C. for 10 minutes, and were cooled at room temperature. Then, the proteins were incubated in a reaction buffer, containing 5 □ of PNGase F (Sigma, Germany), at 37° C. for 3 hours. The deglycosylated proteins were loaded onto SDS-PAGE to separate the samples and were subjected to Coomassie brilliant straining. The SDS-PAGE results are shown in FIG. 2C.
[0093] In-Gel Digestion
[0094]The gel Coomassie-stained in Example was cleaved, and the resulting protein bands were desalted by treatment with 75 mM ammonium bicarbonate / 40% ethanol (1:1). After desalting, a sufficient a...
example 3
LC-ESI-MS / MS Analysis
[0095] LC-ESI-MS / MS
[0096]The peptide mixture produced in Example 2 was analyzed in the following manner using LC-MS / MS. The LC-MS / MS was performed using a Thermo Finnigan's ProteomeX workstation LTQ linear ion trap MS (Thermo Electron, San Jose, Calif., USA) equipped with nonospray ionization sources (NSI sources, San Jose, Calif.). 12 □ of the peptide mixture was injected and loaded into a peptide trap cartridge (Agilent, Palo Alto, Calif.). The trapped peptide was eluted using a 10-cm reversed-phase PicoFrit column (5 □, 300 Å diameter C18) packed in a housing and was separated by gradient elution in a reverse phase column (RP column). As the mobile phase, each of solutions A (H2O) and B (acetonitrile, ACN) was used, and the solutions all contained 0.1% (v / v) formic acid. The flow rate was maintained at 200 nL / min.
[0097]The gradient elution started with 2% mobile phase, and linear gradient elution was performed such that the mobile phase reached 60% within 50 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current | aaaaa | aaaaa |
| Atomic weight | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com