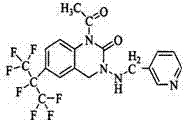
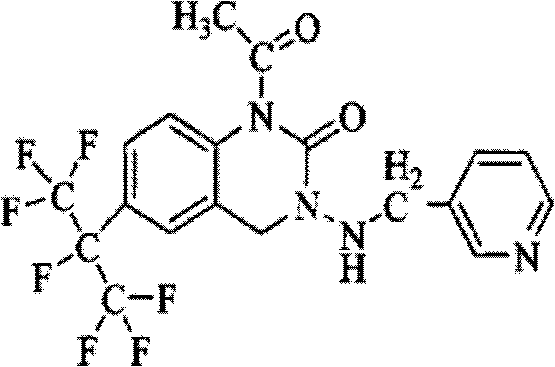
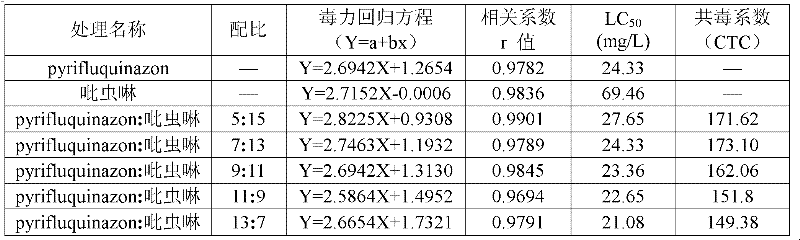
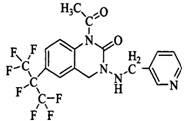
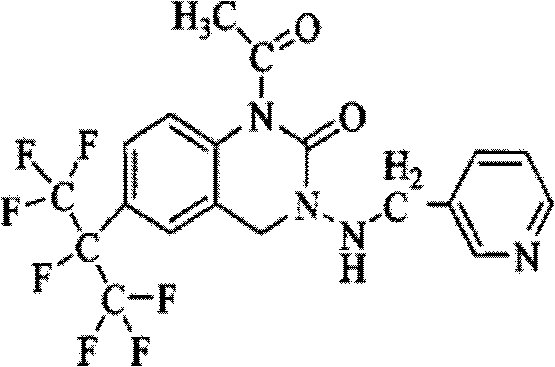
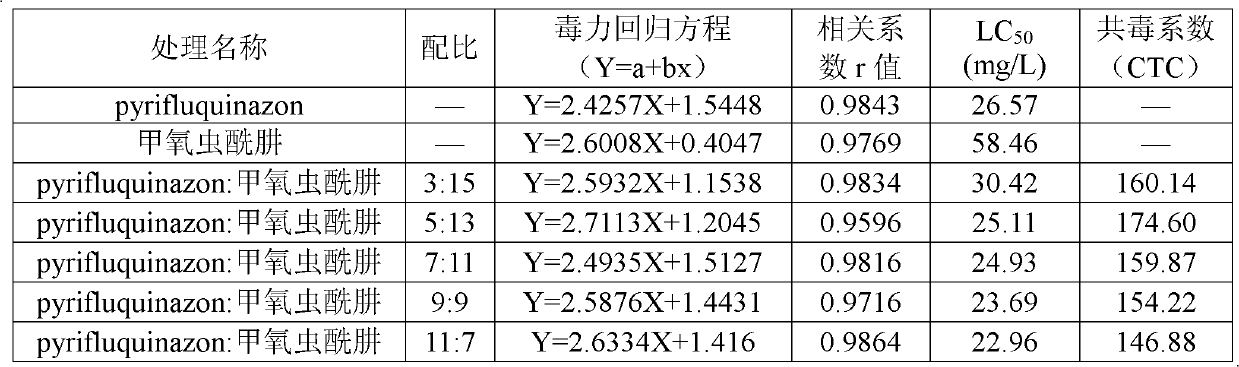
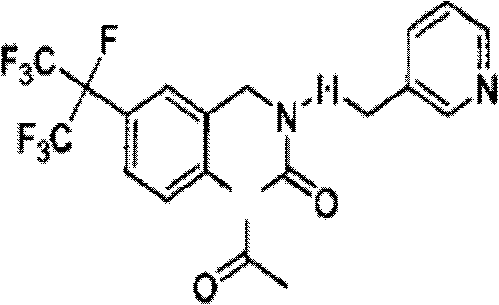
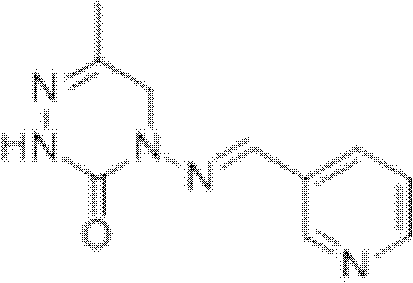
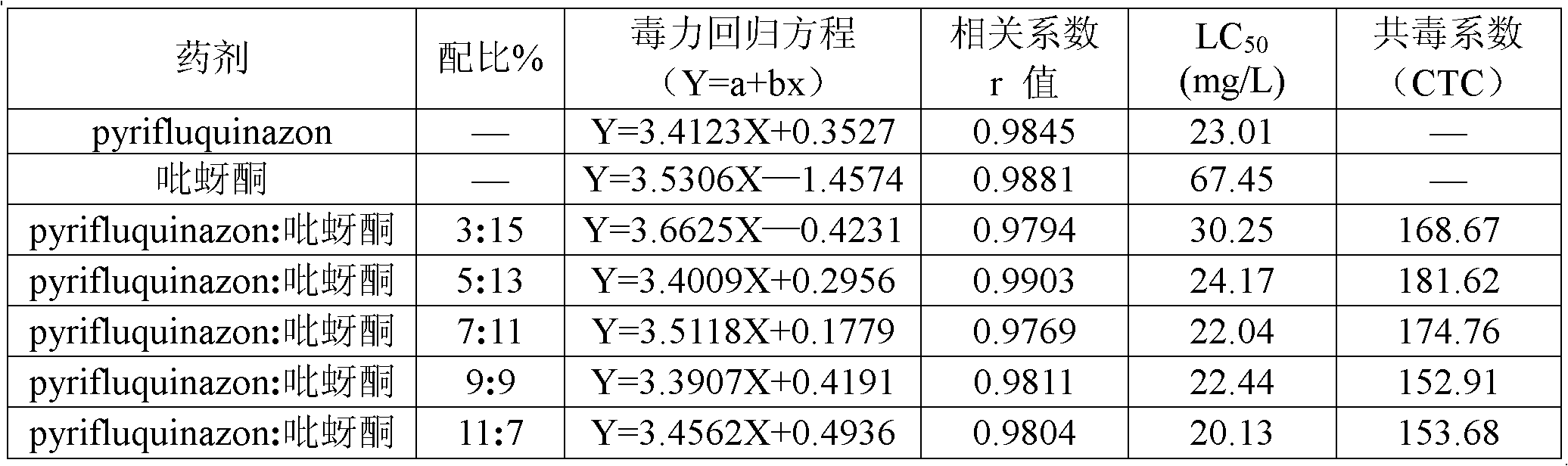
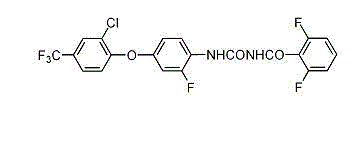
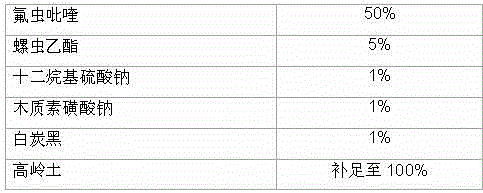
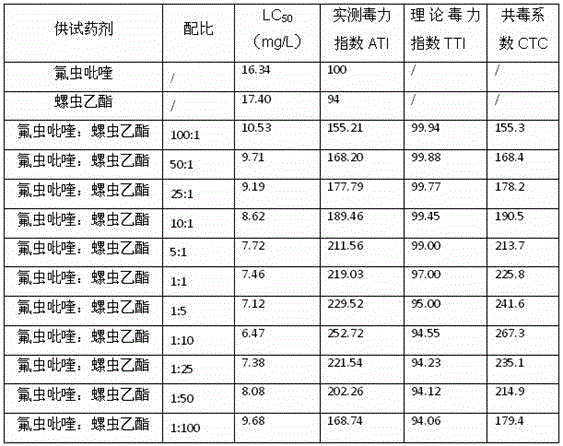
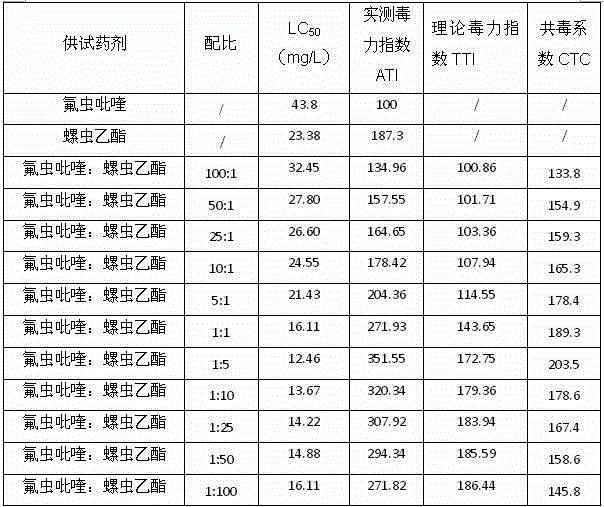
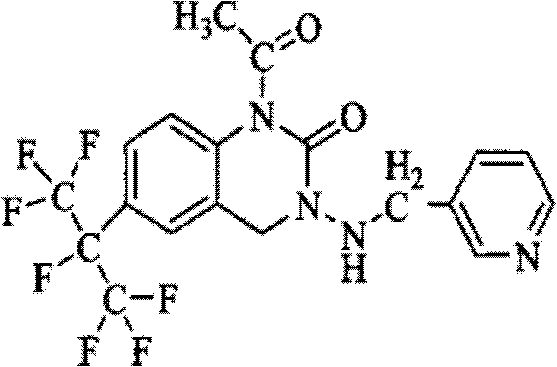
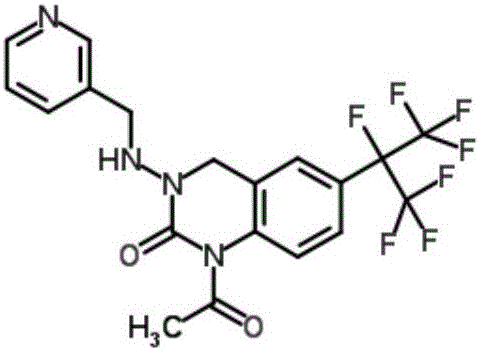
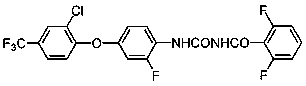
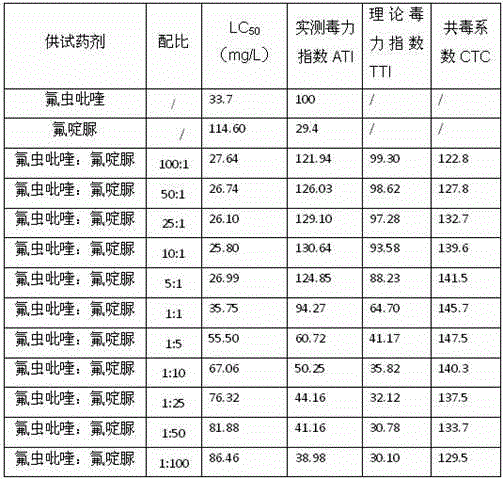
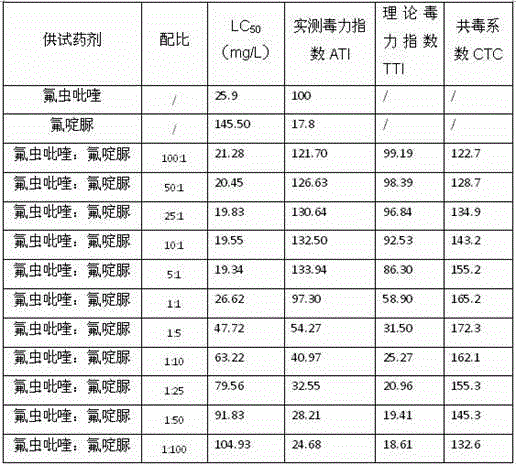
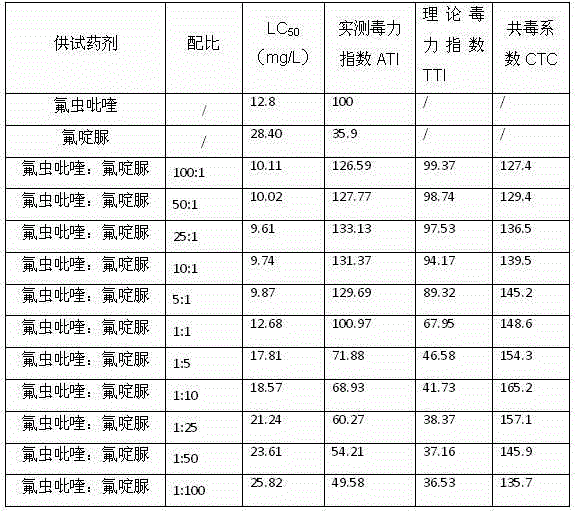
Patents
Literature
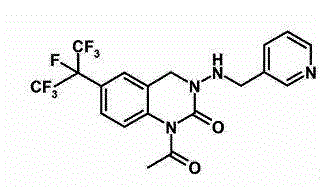
46 results about "Pyrifluquinazon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
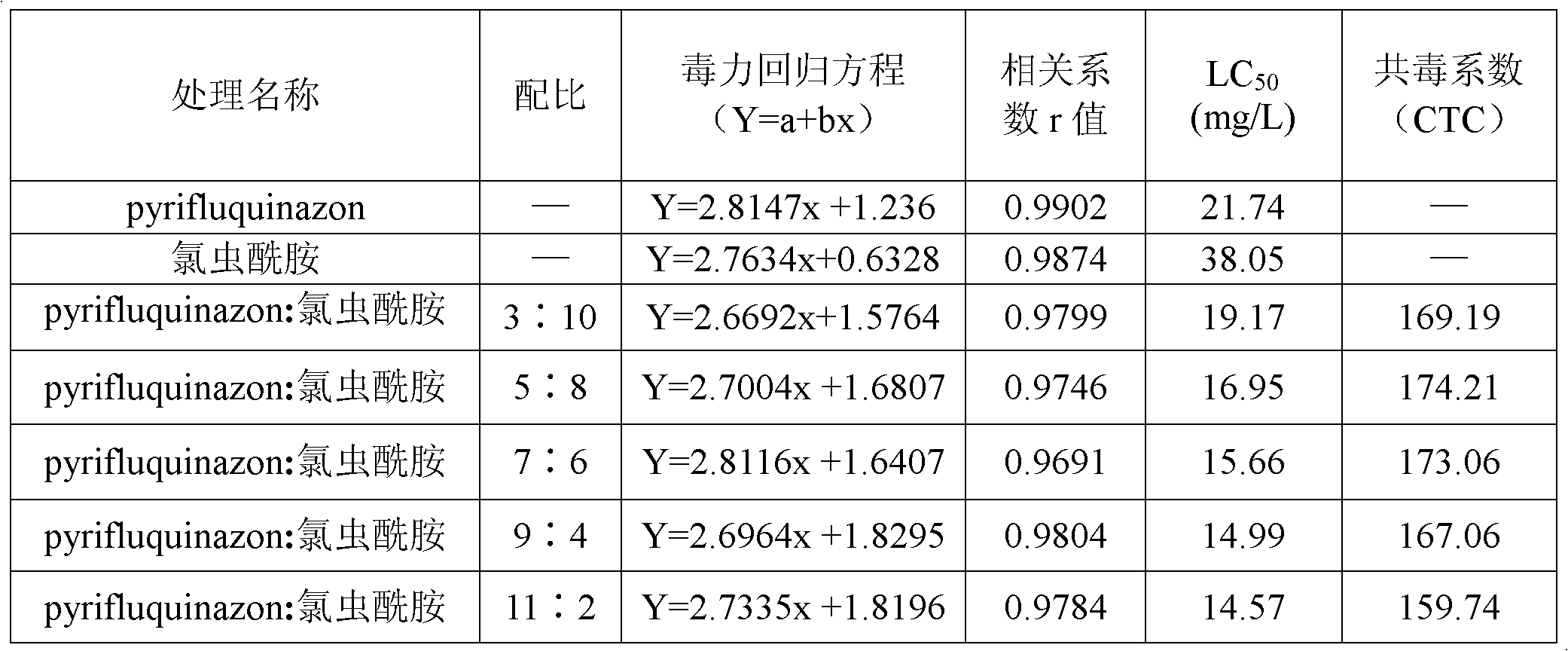
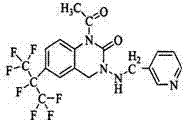
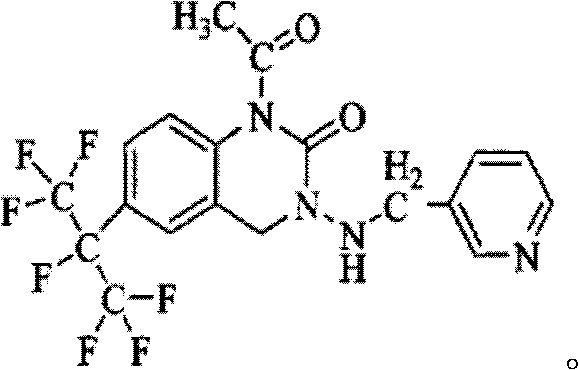
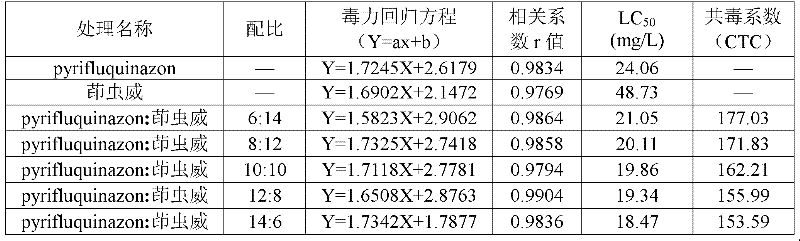
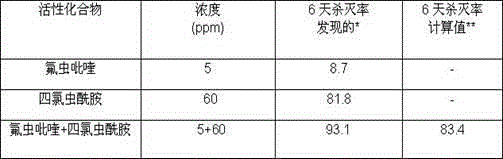
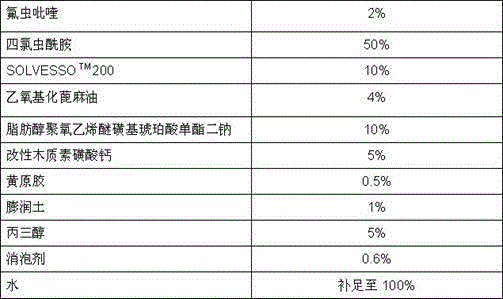
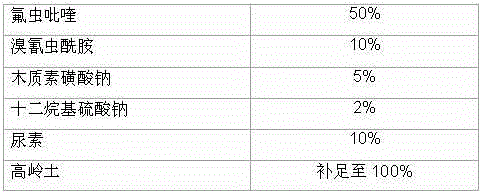
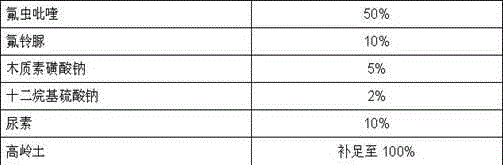
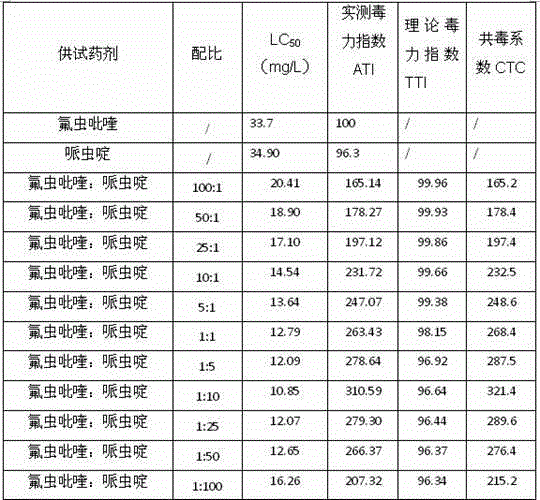
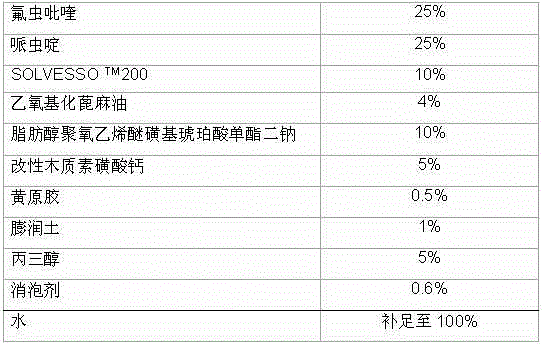
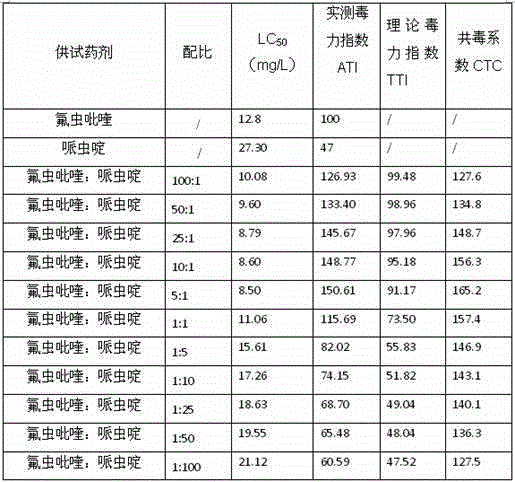
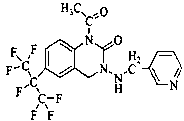
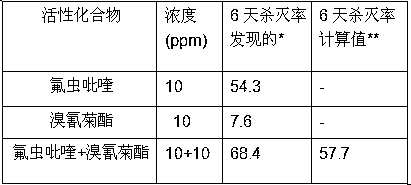
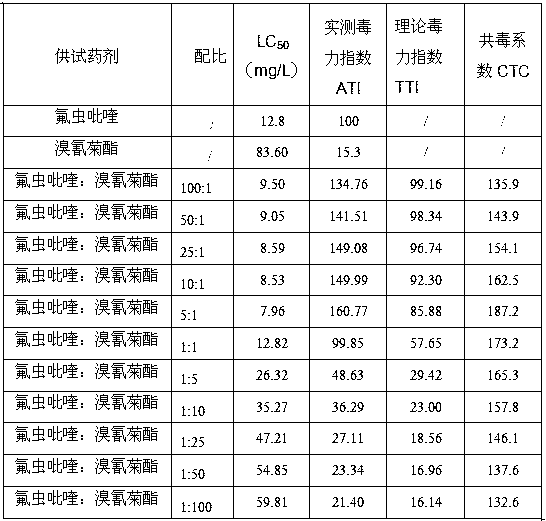
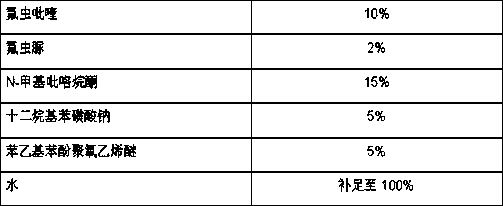
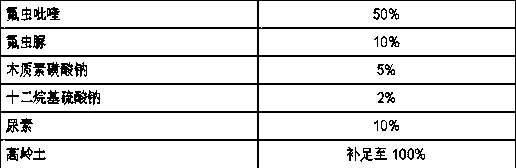
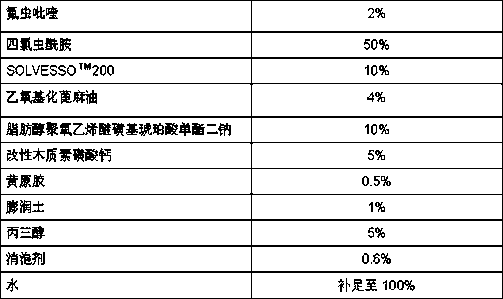
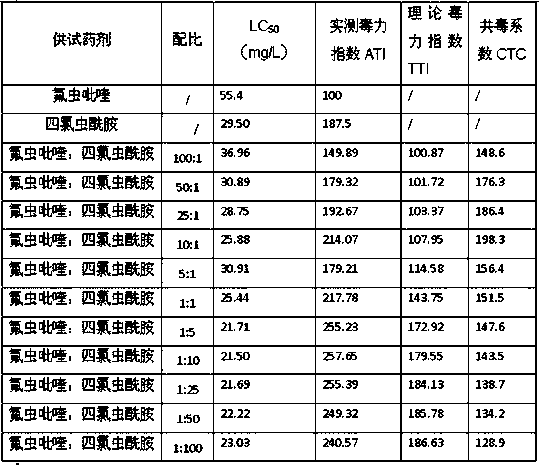
Pesticide composition compounded with part of nicotine compounds
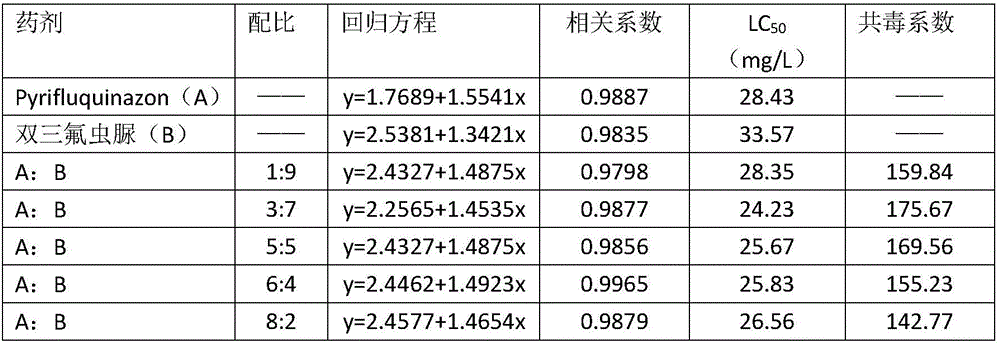
InactiveCN102440257ADelay drug resistanceSynergistic effect is obviousBiocideAnimal repellantsWater dispersibleActive component
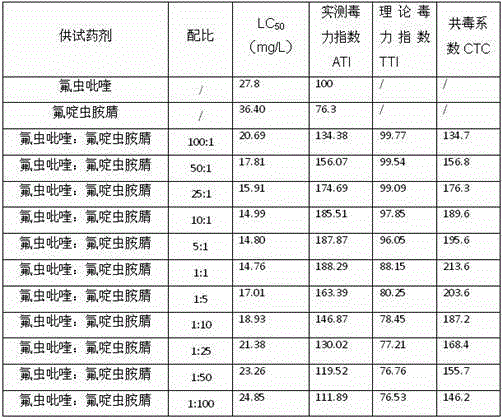
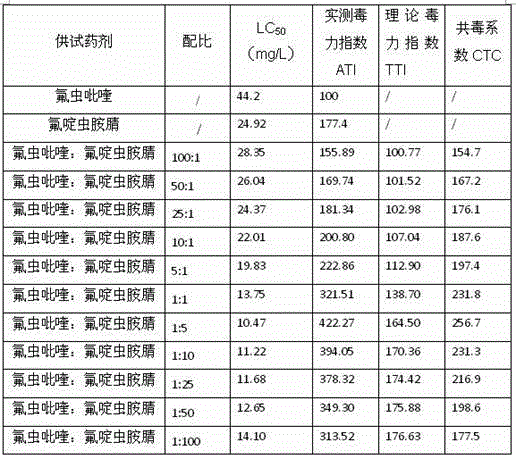
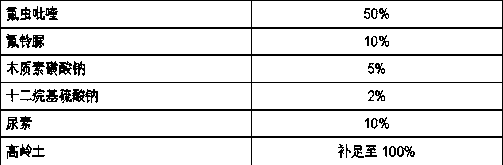
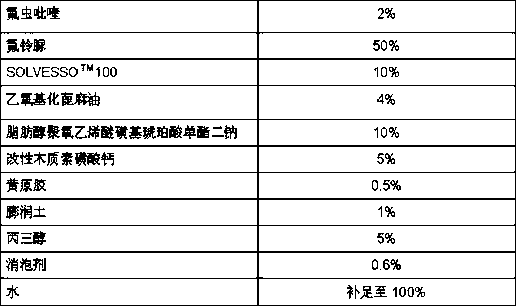
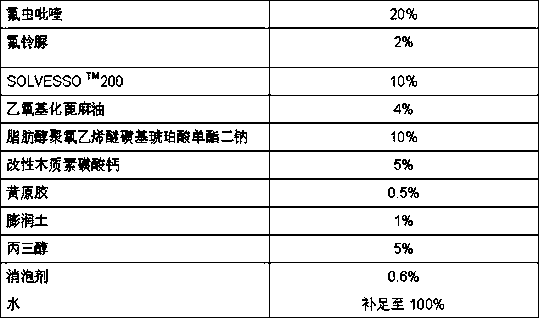
The invention relates to a pesticide composition compounded with part of nicotine compounds in the field of pesticide compounding. The active components of the pesticide composition are novel quinazoline pesticide pyrifluquinazon and part of the nicotine compound pesticides, the weight percentage ratio of the two active components is 1 to 90:90 to 1, preferably 1 to 50:60 to 5, the total mass percentage content of the active components in the pesticide composition is 1 to 90 percent, preferably 5 to 65 percent, the balance is acceptable auxiliary components allowed to be used in pesticides, and a known method is used for preparing the pesticide composition into emulsion in water, soluble concentrate, microcapsule suspension, microemulsion, emulsifiable concentrate, suspension, wettable powder, aqueous solution and water-dispersible granules. The pesticide composition with the novel quinazoline pesticide and the nicotine compound pesticides as the active components is mainly used for controlling pests on citrus trees, other fruit trees, vegetables and tea leaves.
Owner:HAILIR PESTICIDES & CHEM GRP
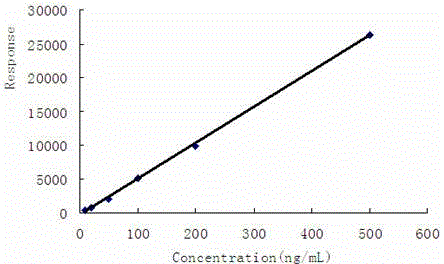
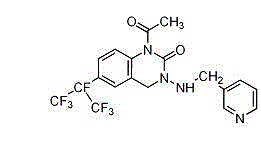
Pyrifluquinazon residue determination method
ActiveCN103728407AAvoid matrix interferenceSimple and fast operationComponent separationEthylenediamineMatrix solution
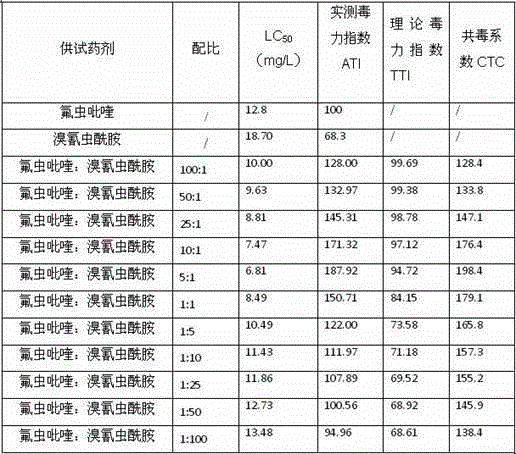
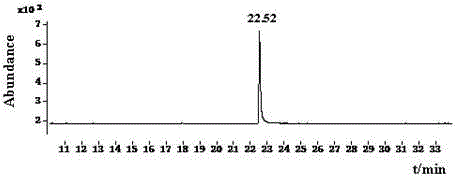
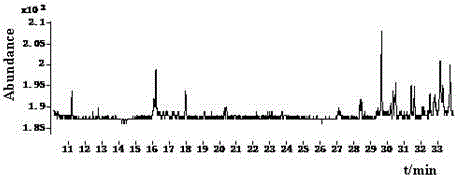
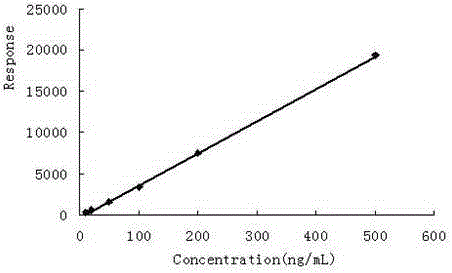
The invention discloses a Pyrifluquinazon residue determination method. The method comprises the steps of homogenously extracting residual Pyrifluquinazon in a sample through acetonitrile or 1% acetic acid acetonitrile, dispersing and purifying an extracting solution through ethylenediamine-N-propyl silicane (PSA), octadecyl silicane bonded phase (C18) and graphitized carbon (Carb) substrate, implementing liquid chromatogram-tandem mass spectrum (LC-MS / MS) detection, establishing a correction standard curve through a blank matrix solution dilution standard, and quantifying through an external standard method. According to the determination method disclosed by the invention, average recovery rate is 85.3-92.0%, average relative standard deviation (RSD) is 3.5-6.1% and detection limit is lower than 0.38 mu g / kg, and the method has the advantages of simplicity and rapidness in operation, high sensitivity, good repeatability, and accuracy in qualitative and quantitative determination. The method can satisfy technical requirements of Korea, Japan, European Union and other countries on corresponding product safety detection, and provide powerful technical support for guaranteeing food safety of Chinese people and healthy development of export trade.
Owner:崔淑华
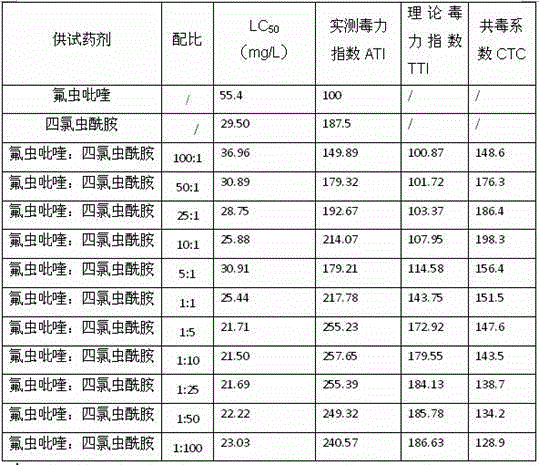
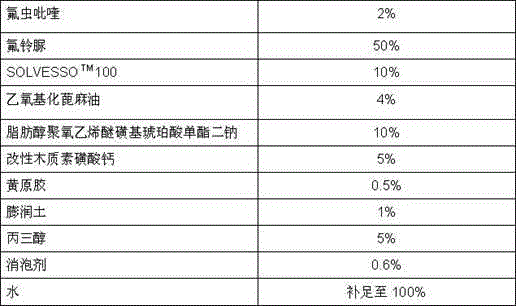
Insecticide composition mixed with amides pesticide
InactiveCN103125493AGood control effectHigh insecticidal activityBiocideAnimal repellantsHomopteraAdditive ingredient
The invention relates to insecticide composition in the field of pesticide mixture. The effective constituent of the insecticide composition mixed with amides pesticide is formed by mixture of novel pyrifluquinazon and one of four amide compounds, wherein the weight ratio of the novel pyrifluquinazon to the one of the four amide compounds is 1-90:90-1, and a preferential weight ratio is 1-45:60-5. Mass ration of the effective constituent of the insecticide composition mixed with amides pesticide is 1%-90% and a preferential mass ration is 5%-65%. The other composition of the insecticide composition mixed with amides pesticide is auxiliary component which is allowed and acceptable in pesticide. The insecticide composition mixed with amides pesticide can be prepared into emulsion in water, microemulsion, missible oil, suspending agents, wettable powder, micro-capsule suspending agents, and water dispersible granule. Due to the fact that the effective constituent of the insecticide composition is formed by the mixture of the pyrifluquinazon and the one of the amides compounds, the insecticide composition mixed with the amides pesticide can effectively prevent lepidopteron, such as plutella xylostella, beet armyworms, prodenia litura, cotton bollworms and mealybug and homoptera piercing-sucking mouthpart insects, such as aphids, aleyrodidaes, and leafhopper.
Owner:HAILIR PESTICIDES & CHEM GRP
Indoxacarb-containing high-efficiency pesticidal composition
InactiveCN102415394AImprove quicknessReduce the use effectBiocideAnimal repellantsCotton bollwormSuspending Agents
The invention relates to a pesticidal composition in the field of pesticide compounding, and discloses a binary mixed pesticidal composition containing active ingredients of a novel quinazoline pyrifluquinazon and indoxacarb. The weight percentage ratio of the novel quinazoline pyrifluquinazon to the indoxacarb is (1-90):(90-1), and (3-50):(60-5) preferably; the sum of the active ingredients is 1 to 90 mass percent of the pesticidal composition, and 5 to 60 percent preferably, and the balance is acceptable auxiliary elements allowed to be used in pesticides; and the pesticidal composition can be prepared into emulsion in water, microemulsion, an emulsifiable solution, a suspending agent, wettable powder and water dispersible granules. The pesticidal composition is mainly used for controlling injurious insects such as cnaphalocrocis medinalis, plutella xylostella, prodenia litura, cotton bollworm, beet armyworm and the like.
Owner:HAILIR PESTICIDES & CHEM GRP
Diacylhydrazine pesticide compounded pesticidal composition
InactiveCN102428922ASynergistic effect is obviousReduce dosageBiocideAnimal repellantsHomopteraOrder Lepidoptera
The invention relates to a pesticidal composition in the field of pesticide compounding. Active ingredients, namely a novel quinazoline pesticide of pyrifluquinazon and a diacylhydrazine pesticide, are subjected to binary mixing, and the weight percentage ratio of the two active ingredients is 1:90-90:1, preferably (5-50):(60-5); and the pesticidal composition contains 1 to 90 mass percent of active ingredients, preferably 5 to 60 percent and the balance of auxiliary elements which are allowed to be used and acceptable in pesticides, and can be prepared into missible oil, a suspending agent, wettable powder, water dispersible granules, emulsion in water, and microemulsion. After the novel quinazoline pesticide of pyrifluquinazon and the diacylhydrazine pesticide are subjected to binary mixing, the pesticidal composition has an obvious control effect on lepidoptera such as plutella xylostella, beet armyworm, prodenia litura, cotton bollworm, striped rice borer, mealybugs and the like, and sucking mouthparts pests of homoptera, such as plant hopper, aphid, mealywing, leafhopper and the like.
Owner:HAILIR PESTICIDES & CHEM GRP
Novel insecticide composition compounding pymetrozine
InactiveCN103125496ADelay drug resistanceSynergistic effect is obviousBiocideAnimal repellantsOptimal weightAphididae
The invention relates to insecticide composition in the field of combination of pesticides. Effective constituents of the insecticide composition comprise novel quinazoline insecticide pyrifluquinazon and pymetrozine, wherein the novel quinazoline insecticide pyrifluquinazon and the pymetrozine are mixed. The weight percentage of the novel quinazoline insecticide pyrifluquinazon and the pymetrozine is 1-90 to 90-1, and the optimal weight percentage is 5-70 to 75-5. The whole quantity percentage of the effective constituents in the insecticide composition is from 1% to 90%, and optimal percentage is from 6% to 70%. The balance is auxiliary elements permitted and accepted in pesticides. Emulsion in water, missible oil, suspending agent, wettable powder and water dispersible granule can be prepared by using a known method. The quinazoline insecticide is mainly used for preventing and curing the pests including aphididae, aleyrodidae, cicadellidae, and delphacidae.
Owner:JIANGXI HAIKE RUITE CROP SCI
Determining method of Pyrifluquinazon residual quantity
ActiveCN104597189AAvoid matrix interferenceSimple and fast operationComponent separationSilanesRelative standard deviation
The invention relates to a determining method of Pyrifluquinazon residual quantity. The method comprises the following steps: homogeneously extracting residual Pyrifluquinazon in a sample by using acetonitrile or an acetonitrile solution containing 1% acetic acid; after dispersed purification of an extracting liquid by using an ethidene diamine-N-propyl silane (PSA) and octadecyl silane bonded phase (C18) matrix, detecting by gas chromatography-negative chemical ionization-chromatography (GC-NCI-MS); and establishing a corrected standard curve by using a blank substrate solution without to-be-detected pesticides, and quantifying by an external standard method. According to the method provided by the invention, the average recovery rate is 88.7-96.1%, the average relative standard deviation (RSD) is 4.7-6.9% and the detection limit is lower than 0.81 microg / kg. The determining method has the advantages of being simple and convenient to operate, quick, high in sensitivity, good in repeatability and accurate to qualify and quantify and can satisfy the technical requirement on safety detection of corresponding products in countries such as Korea, Japan and European Union, and thus a powerful technical support for guaranteeing the food safety of people in China and healthy development of foreign export trade is provided.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
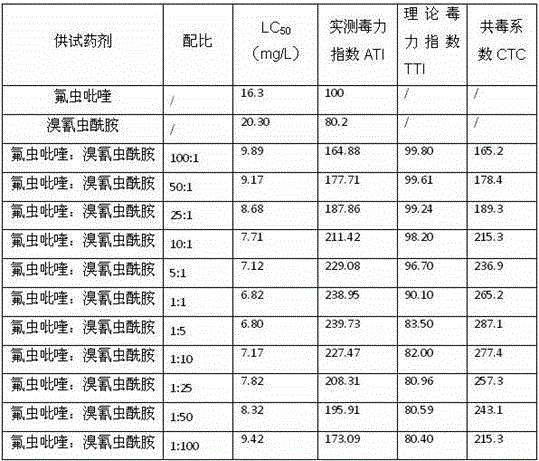
Pyrifluquinazon and clothianidin pesticide composition
InactiveCN105454277ASynergistic effect is obviousLong lastingBiocideDead animal preservationSuspending AgentsToxicology
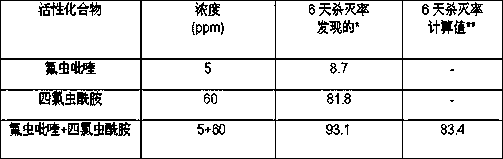
The invention discloses pyrifluquinazon and clothianidin pesticide composition. Effective components of the composition comprise a first active component pyrifluquinazon, a second active component clothianidin and an acceptable dilute or carrier for a pesticide, wherein a weight ratio of the first active component to the second active component is 20:1-1:20, and the best weight ratio of the first active component to the second active component is 5:15-20:1. Agriculturally allowable wettable powder, suspending agents or water dispersible granules can be prepared from the composition. The composition comprises reasonable components, has a better control effect on tomato bemisia tabaci or tea tree empoasca flavescens matumura as well as low application cost, long persistence, small preparation residues and good safety and is non-pollution for crops, and besides, compared with a conventional single agent, the composition can effectively slow down resistance and meets the safety requirements of pesticide preparations.
Owner:SHAANXI BIAOZHENG CROP SCIENCE CO LTD
Insect disinfestation composition and pest control method
ActiveCN104904741AImprove long-term effectExpand insecticidal spectrumBiocideAnimal repellantsBiotechnologyPlanting seed
The present invention relates to a pesticidal composition comprising pyrifluquinazon and lambda-cyhalothrin, and specifically relates to a method for controlling pests. The pesticidal composition comprising pyrifluquinazon and lambda-cyhalothrin has a weight ratio of pyrifluquinazon to lambda-cyhalothrin of 1:100 to 100:1. A method for preventing or controlling pests: using the pesticidal composition on a target useful plant, a target pest or an environment thereof, and a reproductive material for the target useful plant. A method for protecting plant seeds comprising: allowing seeds to be in contact with an efficacious dose of the pesticidal composition of the present invention before sowing and / or after germination. A seed administered and treated with an efficacious dose of the pesticidal composition of the present invention.
Owner:JIANGSU ROTAM CHEM
Insect disinfestation composition and pest control method thereof
The present invention relates to a pesticidal composition, comprising pyrifluquinazon and ethofenprox, and specifically relates to a method for controlling pests. The pesticidal composition comprising pyrifluquinazon and ethofenprox has a weight ratio of pyrifluquinazon to ethofenprox of 1:100 to 100:1. A method for preventing or controlling pests: using the pesticidal composition on a target useful plant, a target pest or an environment thereof, and a reproductive material for the target useful plant. A method for protecting plant seeds, comprising: allowing seeds to be in contact with an efficacious dose of the pesticidal composition of the present invention before sowing and / or after germination. A seed administered and treated with an efficacious dose of the pesticidal composition of the present invention.
Owner:JIANGSU ROTAM CHEM
Insecticidal composition and agricultural insect control method
ActiveCN105794788ASynergistic effect is obviousReduce dosageBiocideNematocidesPlanting seedSeed treatment
The invention relates to an insecticidal composition. The insecticidal composition comprises pyrifluquinazon and SYP-9080. The invention especially relates to an agricultural insect control method. A weight ratio of pyrifluquinazon to SYP-9080 is 1: 100 to 100: 1. The invention discloses a method for preventing or controlling agricultural insects. The method utilizes the insecticidal composition for a target useful plant, a target agricultural insect or its environment, and a target useful plant propagating material. The invention discloses a method for preventing agricultural insect attack. The invention discloses a plant seed protection method and the method comprises that seeds contact with an effective amount of the insecticidal composition before and / or after pregermination. The insecticidal composition is used for seed treatment.
Owner:JIANGSU ROTAM CHEM
Insecticidal composition and agricultural insect control method
ActiveCN105794787ASynergistic effect is obviousReduce dosageBiocideNematocidesPlanting seedSeed treatment
The invention relates to an insecticidal composition. The insecticidal composition comprises pyrifluquinazon and cyantraniliprole. The invention especially relates to an agricultural insect control method. A weight ratio of pyrifluquinazon to cyantraniliprole is 1: 100 to 100: 1. The invention discloses a method for preventing or controlling agricultural insects. The method utilizes the insecticidal composition for a target useful plant, a target agricultural insect or its environment, and a target useful plant propagating material. The invention discloses a plant seed protection method and the method comprises that seeds contact with an effective amount of the insecticidal composition before and / or after pregermination. The insecticidal composition is used for seed treatment.
Owner:JIANGSU ROTAM CHEM
Insecticidal composition and agricultural insect control method
The invention relates to an insecticidal composition. The insecticidal composition comprises pyrifluquinazon and hexaflumuron. The invention especially relates to an agricultural insect control method. A weight ratio of pyrifluquinazon to hexaflumuron is 1: 100 to 100: 1. The invention discloses a method for preventing or controlling agricultural insects. The method utilizes the insecticidal composition for a target useful plant, a target agricultural insect or its environment, and a target useful plant propagating material. The invention discloses a method for preventing agricultural insect attack. The method comprises utilizing the insecticidal composition for plants, a plant propagating material and an environment before and / or after insect attack.
Owner:JIANGSU ROTAM CHEM
Insecticidal composition and insect control method
ActiveCN105794828ASynergistic effect is obviousReduced dose rateBiocideNematocidesToxicologySulfoxaflor
The invention relates to an insecticidal composition. The insecticidal composition comprises pyrifluquinazon and sulfoxaflor. The invention especially relates to an insect control method. A weight ratio of pyrifluquinazon to sulfoxaflor is 1: 100 to 100: 1. The invention discloses a method for preventing or controlling insects. The method utilizes the insecticidal composition for a target useful plant, a target agricultural insect or its environment, and a target useful plant propagating material. The invention discloses a method for preventing plants from being attacked by insects. The method comprises utilizing the insecticidal composition for plants, a plant propagating material and an environment before and / or after insect attack. The invention discloses a use of the insecticidal composition in prevention or control of sanitation pests.
Owner:JIANGSU ROTAM CHEM
Insecticidal composition and agricultural insect control method
ActiveCN105794814ASynergistic effect is obviousReduced dose rateBiocideAnimal repellantsToxicologyBotany
The invention relates to an insecticidal composition. The insecticidal composition comprises pyrifluquinazon and flufenoxuron. The invention especially relates to an agricultural insect control method. A weight ratio of pyrifluquinazon to flufenoxuron is 1: 100 to 100: 1. The invention discloses a method for preventing or controlling agricultural insects. The method utilizes the insecticidal composition for a target useful plant, a target agricultural insect or its environment, and a target useful plant propagating material. The invention discloses a method for preventing agricultural insect attack. The method comprises utilizing the insecticidal composition for plants, a plant propagating material and an environment before and / or after insect attack.
Owner:JIANGSU ROTAM CHEM
Insecticidal composition and agricultural insect control method
InactiveCN105794804AReduce the number of medicationsReduce labor costsBiocideAnimal repellantsPlanting seedSpirotetramat
The invention relates to an insecticidal composition. The insecticidal composition comprises pyrifluquinazon and spirotetramat. The invention especially relates to an agricultural insect control method. A weight ratio of pyrifluquinazon to spirotetramat is 1: 100 to 100: 1. The invention discloses a method for preventing or controlling agricultural insects. The method utilizes the insecticidal composition for a target useful plant, a target agricultural insect or its environment, and a target useful plant propagating material. The invention discloses a plant seed protection method and the method comprises that seeds contact with an effective amount of the insecticidal composition before and / or after pregermination. The insecticidal composition is used for seed treatment.
Owner:JIANGSU ROTAM CHEM
Insecticidal composition
ActiveCN105794798AExpand insecticidal spectrumImprove long-term effectBiocideNematocidesPaichongdingPlanting seed
The invention relates to an insecticidal composition. The insecticidal composition comprises pyrifluquinazon and Paichongding. The invention especially relates to an agricultural insect control method. The insecticidal composition comprises pyrifluquinazon and Paichongding. A weight ratio of pyrifluquinazon to Paichongding is 1: 100 to 100: 1. The invention discloses a method for preventing or controlling agricultural insects. The method utilizes the insecticidal composition for a target useful plant, a target agricultural insect or its environment, and a target useful plant propagating material. The invention discloses a method for protecting plant seeds. The method comprises that seeds contact with an effective amount of the insecticidal composition before and / or after pregermination. The insecticidal composition is used for seed treatment.
Owner:JIANGSU ROTAM CHEM
Insecticidal composition compounded with macrolide compound
InactiveCN103155930ASynergistic effect is obviousDelay drug resistanceBiocideAnimal repellantsAdditive ingredientAbamectin
The present invention relates to an insecticidal composition, and belongs to the field of insecticidal compounding. The active ingredients include novel quinazoline insecticidal pyrifluquinazon and abamectin or emamectin benzoate. The weight ratio of the two active ingredients is 1-90:50-0.1, and preferably 5-50:20-0.1. The mass percentage of the total amount of active ingredients in the insecticidal composition is 1%-90%, preferably 1% -40%, and the rest is insecticide allowed and acceptable auxiliary components. The insecticidal composition can be prepared into water emulsion, micro-emulsion, missible oil, a suspending agent, wettable powder, granules, soluble liquid, water dispersible granule, and a microcapsule suspending agent by known methods. The insecticidal composition can be used to control insects of aphids, whiteflies, leafhoppers and planthoppers, is suitable for vegetables, ornamental plants, fruit trees, citrus, rice, cotton and multiple field crops.
Owner:HAILIR PESTICIDES & CHEM GRP
Pyrifluquinazon and bistrifluron compound composition
InactiveCN106212489AReduce pollutionImprove efficiencyBiocideDead animal preservationPesticide residueSuspending Agents
The invention discloses a Pyrifluquinazon and bistrifluron compound composition, the effective ingredients of the compound composition are Pyrifluquinazon and bistrifluron, and the weight percentage of the Pyrifluquinazon and the bistrifluron in the compound composition is (5-50):(50-5), preferably (5-20):(40-5). The dosage forms which can be prepared by the composition are emulsion in water, water dispersible granules, microemulsion, a suspending agent, wet powder and missible oil, and the total weight of the Pyrifluquinazon and the bistrifluron in the dosage forms rakes up 5%-80% of the total weight of the whole preparation, preferably 10%-60%. According to the composition, the mechanisms of action are different, the synergistic effect is remarkable after compounding, the pest resistance to insecticide is delayed, the insecticidal spectrum is widened, particularly importantly the effective drug dose per unit area is significantly reduced, the pesticide residue is reduced, and the pesticide pollution is alleviated.
Owner:YANCHENG INST OF TECH
A kind of insecticidal composition and method for controlling agricultural pests
The invention relates to an insecticidal composition. The insecticidal composition comprises pyrifluquinazon and hexaflumuron. The invention especially relates to an agricultural insect control method. A weight ratio of pyrifluquinazon to hexaflumuron is 1: 100 to 100: 1. The invention discloses a method for preventing or controlling agricultural insects. The method utilizes the insecticidal composition for a target useful plant, a target agricultural insect or its environment, and a target useful plant propagating material. The invention discloses a method for preventing agricultural insect attack. The method comprises utilizing the insecticidal composition for plants, a plant propagating material and an environment before and / or after insect attack.
Owner:JIANGSU ROTAM CHEM
A kind of insecticidal composition and method for controlling agricultural pests
The invention relates to an insecticidal composition. The insecticidal composition comprises pyrifluquinazon and chlorfluazuron. The invention especially relates to an agricultural insect control method. A weight ratio of pyrifluquinazon to chlorfluazuron is 1: 100 to 100: 1. The invention discloses a method for preventing or controlling agricultural insects. The method utilizes the insecticidal composition for a target useful plant, a target agricultural insect or its environment, and a target useful plant propagating material. The invention discloses a method for preventing agricultural insect attack. The method comprises utilizing the insecticidal composition for plants, a plant propagating material and an environment before and / or after insect attack.
Owner:JIANGSU ROTAM CHEM
A kind of insecticidal composition and method for controlling harmful organisms
ActiveCN104904741BImprove long-term effectExpand insecticidal spectrumBiocideAnimal repellantsBiotechnologyPlanting seed
The present invention relates to a pesticidal composition comprising pyrifluquinazon and lambda-cyhalothrin, and specifically relates to a method for controlling pests. The pesticidal composition comprising pyrifluquinazon and lambda-cyhalothrin has a weight ratio of pyrifluquinazon to lambda-cyhalothrin of 1:100 to 100:1. A method for preventing or controlling pests: using the pesticidal composition on a target useful plant, a target pest or an environment thereof, and a reproductive material for the target useful plant. A method for protecting plant seeds comprising: allowing seeds to be in contact with an efficacious dose of the pesticidal composition of the present invention before sowing and / or after germination. A seed administered and treated with an efficacious dose of the pesticidal composition of the present invention.
Owner:JIANGSU ROTAM CHEM
A kind of insecticidal composition and method for controlling agricultural pests
ActiveCN105794810BSynergistic effect is obviousReduced dose rateBiocideAnimal repellantsAgronomyUseful plants
The invention relates to an insecticidal composition. The insecticidal composition comprises pyrifluquinazon and diafenthiuron. The invention especially relates to an agricultural insect control method. A weight ratio of pyrifluquinazon to diafenthiuron is 1: 100 to 100: 1. The invention discloses a method for preventing or controlling agricultural insects. The method utilizes the insecticidal composition for a target useful plant, a target agricultural insect or its environment, and a target useful plant propagating material. The invention discloses a method for preventing agricultural insect attack. The method comprises utilizing the insecticidal composition for plants, a plant propagating material and an environment before and / or after insect attack.
Owner:JIANGSU ROTAM CHEM
Insecticidal composition of pyrifluquinazon and cyenopyrafen
InactiveCN109744242ASynergistic effect is obviousDelays resistance developmentBiocideAnimal repellantsCyenopyrafenAdditive ingredient
The invention relates to an insecticidal composition of pyrifluquinazon and cyenopyrafen, wherein the weight ratio of two active ingredients is 1: 30-30: 1; the total mass percentage content of the active ingredients in the insecticidal composition is 4-80%; and the rest are auxiliary ingredients which are allowed to be used and acceptable in pesticides, and the composition can be prepared into asuspending agent, an aqueous emulsion, a microemulsion, missible oil, a wettable powder agent, a microcapsule suspending agent, a water dispersible granule, a soluble powder agent and a soluble granule. The composition has obvious synergistic crops, can reduce the utilization rate of pesticides, and is environment-friendly and safe.
Owner:佛山市盈辉作物科学有限公司
A kind of insecticidal composition and method for controlling agricultural pests
ActiveCN105707115BSynergistic effect is obviousReduce dosageBiocideAnimal repellantsBiotechnologyPlanting seed
Owner:JIANGSU ROTAM CHEM
A kind of insecticidal composition and method for controlling agricultural pests
ActiveCN105794814BSynergistic effect is obviousReduced dose rateBiocideAnimal repellantsToxicologyBotany
The invention relates to an insecticidal composition. The insecticidal composition comprises pyrifluquinazon and flufenoxuron. The invention especially relates to an agricultural insect control method. A weight ratio of pyrifluquinazon to flufenoxuron is 1: 100 to 100: 1. The invention discloses a method for preventing or controlling agricultural insects. The method utilizes the insecticidal composition for a target useful plant, a target agricultural insect or its environment, and a target useful plant propagating material. The invention discloses a method for preventing agricultural insect attack. The method comprises utilizing the insecticidal composition for plants, a plant propagating material and an environment before and / or after insect attack.
Owner:JIANGSU ROTAM CHEM
Insecticidal composition and agricultural insect control method
The invention relates to an insecticidal composition. The insecticidal composition comprises pyrifluquinazon and chlorfluazuron. The invention especially relates to an agricultural insect control method. A weight ratio of pyrifluquinazon to chlorfluazuron is 1: 100 to 100: 1. The invention discloses a method for preventing or controlling agricultural insects. The method utilizes the insecticidal composition for a target useful plant, a target agricultural insect or its environment, and a target useful plant propagating material. The invention discloses a method for preventing agricultural insect attack. The method comprises utilizing the insecticidal composition for plants, a plant propagating material and an environment before and / or after insect attack.
Owner:JIANGSU ROTAM CHEM
A kind of assay method of pyrifluquinazon residue
ActiveCN104614478BAvoid matrix interferenceSimple and fast operationComponent separationMatrix solutionRelative standard deviation
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
A kind of insecticidal composition and method for controlling agricultural pests
ActiveCN105794788BSynergistic effect is obviousReduce dosageBiocideNematocidesPlanting seedSeed treatment
The invention relates to an insecticidal composition. The insecticidal composition comprises pyrifluquinazon and SYP-9080. The invention especially relates to an agricultural insect control method. A weight ratio of pyrifluquinazon to SYP-9080 is 1: 100 to 100: 1. The invention discloses a method for preventing or controlling agricultural insects. The method utilizes the insecticidal composition for a target useful plant, a target agricultural insect or its environment, and a target useful plant propagating material. The invention discloses a method for preventing agricultural insect attack. The invention discloses a plant seed protection method and the method comprises that seeds contact with an effective amount of the insecticidal composition before and / or after pregermination. The insecticidal composition is used for seed treatment.
Owner:JIANGSU ROTAM CHEM
Insect disinfestation composition and pest control method
InactiveCN104904742AConvenient amountSolution durationBiocideAnimal repellantsChlorpyrifosPlanting seed
The present invention relates to a pesticidal composition, comprising pyrifluquinazon and chlorpyrifos, and specifically relates to a method for controlling pests. The pesticidal composition comprising pyrifluquinazon and chlorpyrifos has a weight ratio of pyrifluquinazon and chlorpyrifos of 1:100 to 100:1. The method for controlling pests: using the pesticidal composition on a target useful plant, a target pest or an environment thereof, and a reproductive material of the target useful plant. A method for protecting plant seeds, comprising: allowing seeds to be in contact with an efficacious dose of the pesticidal composition of the present invention before sowing and / or after germination. A seed administered and treated with an efficacious dose of the pesticidal composition of the present invention.
Owner:JIANGSU ROTAM CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com