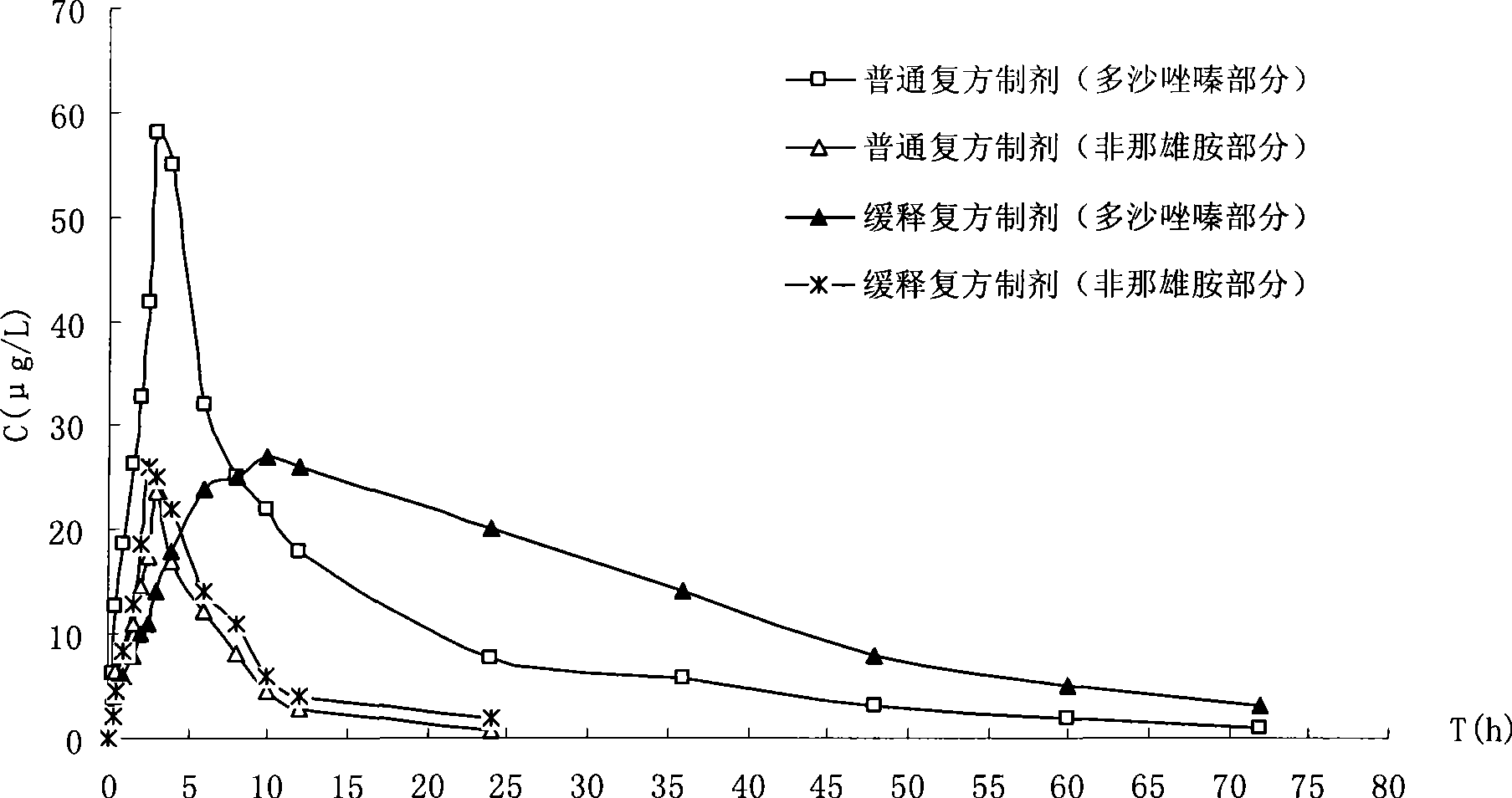
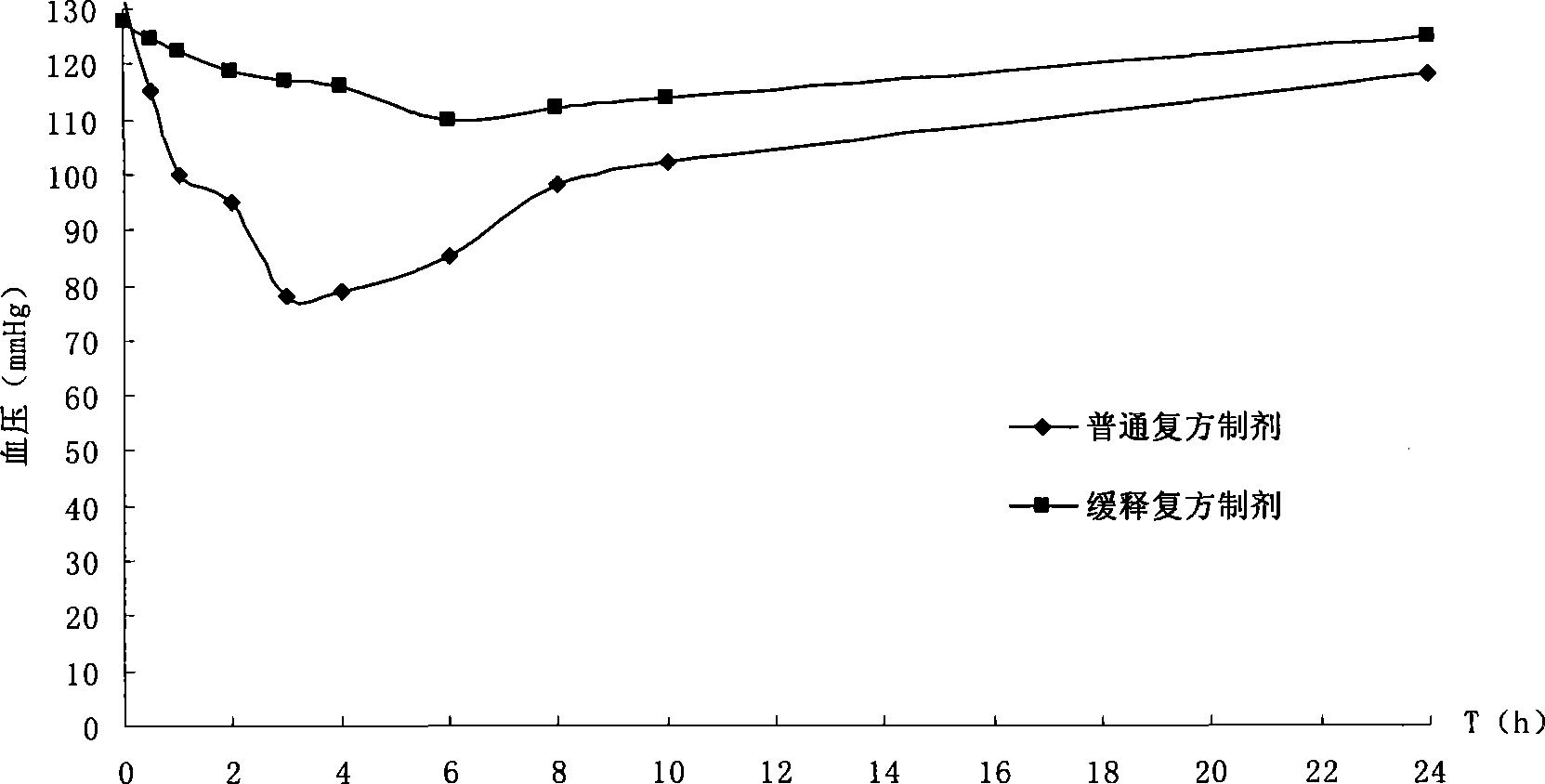
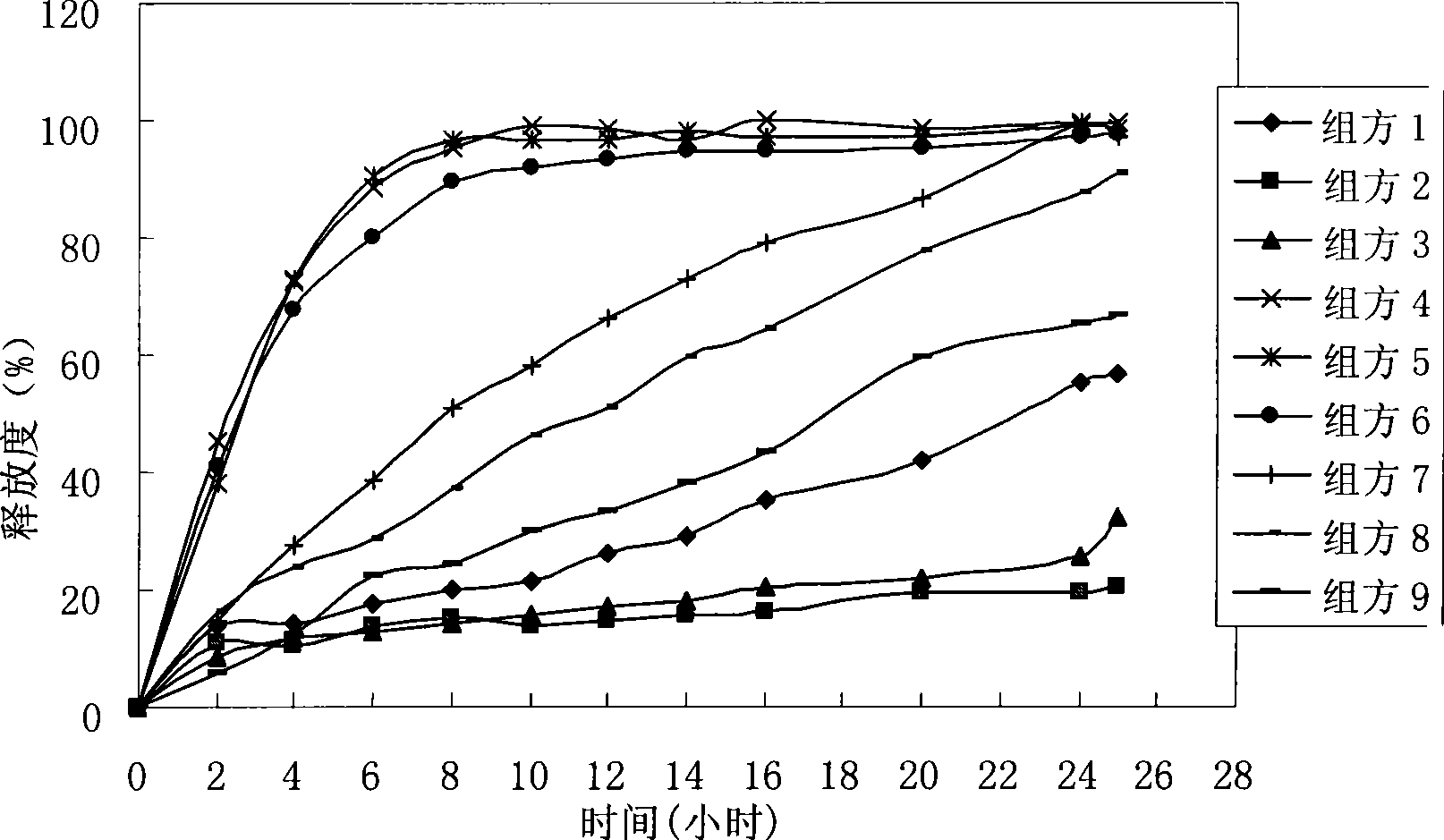
Method for preparing compound controlled release capsule for treating benign prostatic hyperplasia and the compound controlled release capsule
A technology for prostatic hyperplasia and compound prescription, applied in the field of medicine, can solve the problems of lack of controlled release accuracy, inability to effectively control and coordinate the speed and degree of drug release, and unsuitable preparation procedures.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Preparation of finasteride immediate-release pellets
[0040] Finasteride micropellet coating preparation process: mix the drug, binder, co-solvent and organic solvent in the formula to make a coating solution; use the above coating solution to coat the blank sugar pill core in a fluidized bed Coating, during coating, the control 100mg pellets contain 5mg of finasteride.
[0041] Here, the drug is finasteride; the binder can be selected from starch slurry, methylcellulose, hydroxypropylcellulose, hypromellose, ethylcellulose, povidone, gelatin, polyethylene glycol, etc. One or more of them are mixed; the organic solvent can be methanol, ethanol, isopropanol, acetone or any one or more of them mixed or any one or more of them in aqueous solution; when the drug solubility is not enough, A co-solvent can be added, and the co-solvent is selected from sodium lauryl sulfate, Tween, Span, phospholipid, polyoxyethylene polyoxypropylene copolymer, glyceryl stearat...
Embodiment 2
[0065] Embodiment 2: Preparation of doxazosin sustained-release pellets
[0066] 1. The coating preparation process of doxazosin sustained-release pellets: first, mix the drug with fillers and binders, then perform wet granulation, and then extrude and spheronize to prepare drug pellets. After the drug pellets are dried, use a fluidized The bed is coated with a coating liquid, and the weight gain of the coating is 5-17%.
[0067] Here, the drug in the drug pellets is doxazosin or its pharmaceutically acceptable salt, and the pharmaceutically acceptable salt can be hydrochloride, sulfate, mesylate, maleate, preferably doxazosin mesylate; The agent is selected from one or more of starch slurry, methyl cellulose, hydroxypropyl cellulose, hypromellose, ethyl cellulose, povidone, gelatin, polyethylene glycol, etc.; the filler is A mixture of one or more selected from lactose, mannitol, xylitol, sorbitol, powdered sugar, dextrin, starch, pregelatinized starch, microcrystalline cell...
Embodiment 3
[0076] Embodiment 3, the preparation of doxazosin sustained-release pellets
[0077] According to the preferred process of Example 2, the following doxazosin sustained-release pellets were prepared.
[0078] Recipe 1:
[0079] drug pellets
[0080] Doxazosin 4.00g
[0081] Powdered sugar 62.00g
[0082] Starch 24.04g
[0083] Hypromellose 10.0g
[0084] Coating solution
[0085] Ethyl cellulose 10.0g
[0086] Hydroxypropyl methylcellulose 10.0g
[0087] Ethanol 380.0g
[0088] Recipe 2:
[0089] drug pellets
[0090] Doxazosin Mesylate 5.0g
[0091] Powdered sugar 55.0g
[0092] Microcrystalline Cellulose 25.0g
[0093] Hypromellose 15.0g
[0094] Coating solution
[0095] Ethyl cellulose 10.0g
[0096] Hydroxypropyl methylcellulose 10.0g
[0097] Ethanol 380.0g
[0098] Recipe 3:
[0099] drug pellets
[0100] Doxazosin 4.0g
[0101] Lactose 70.0g
[0102] Microcrystalline Cellulose 20.0g
[0103] Povidone 6.0g
[0104] Coating solution
[0105] Ethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com