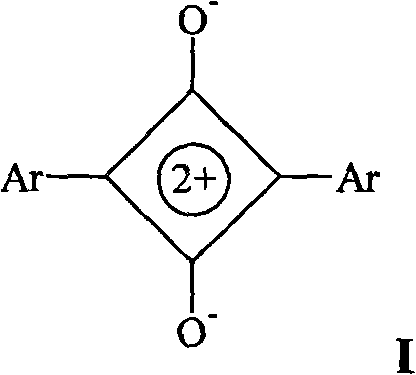
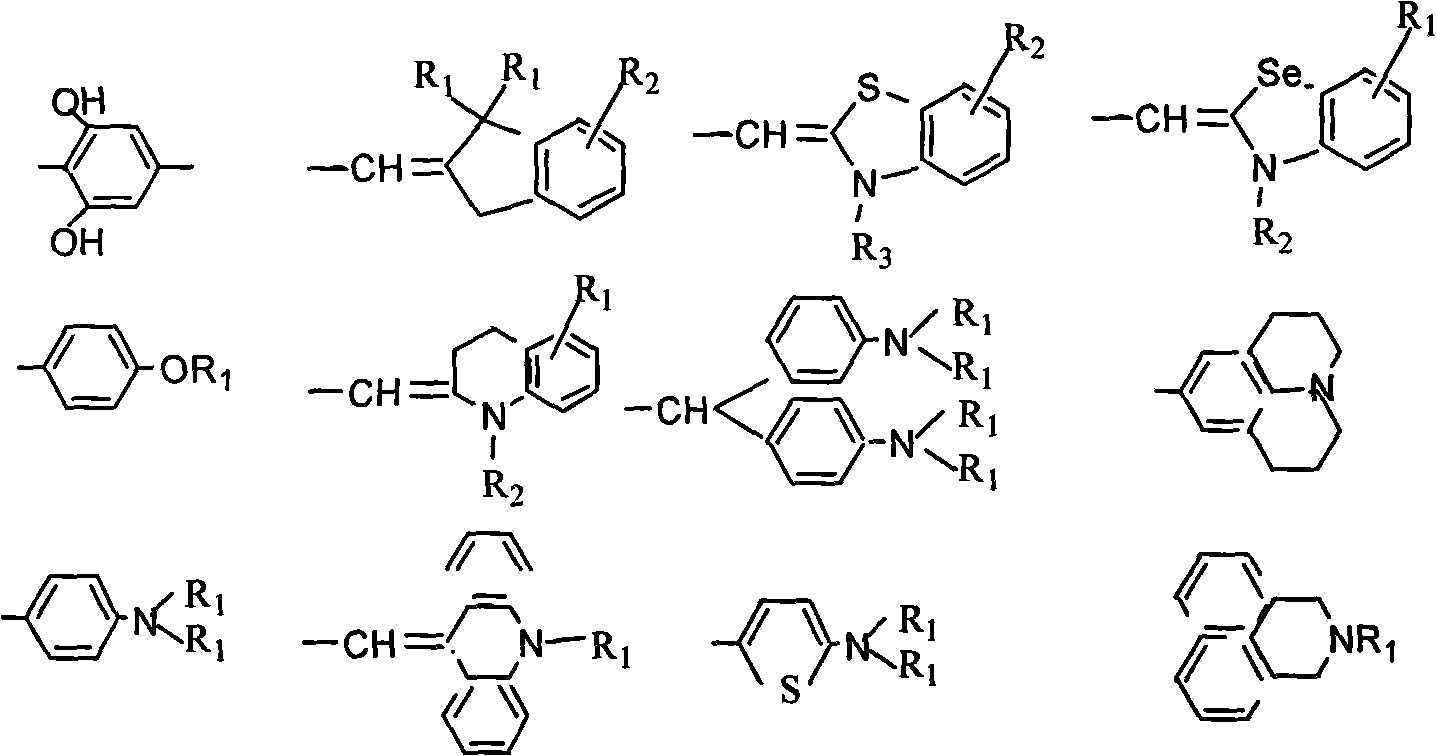
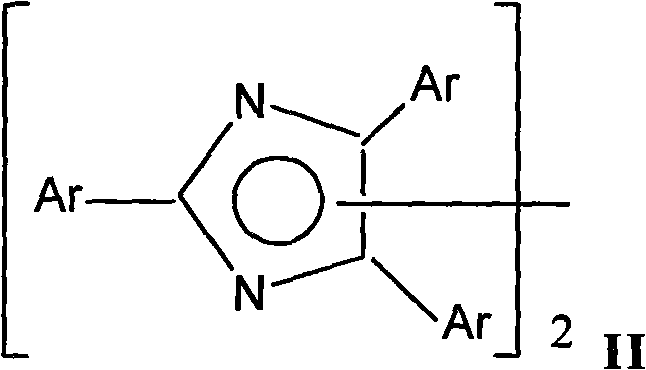
Squaric acid dye/hexaarylbiimidazole complex capable of being used for visible photosensitive system
A technology of hexaarylbisimidazole and compound, which is applied in the field of new compound initiators for visible photosensitive polymerization, and achieves the effect of high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Synthesis of squarylium dye SQ-1 (1,3 bis(p-N,N-trimethylaniline)) squarylium
[0059] N,N-Dimethylaniline 10g (83mmol) squaraine 5g (44mmol) n-butanol 200ml, toluene 200ml reflux dehydration, reacted for 6 hours to produce a blue lake, after drying the product 4.5g yield 31%. The product is identified by infrared spectrum, ultraviolet spectrum, hydrogen nuclear magnetic resonance spectrum and mass spectrum.
Embodiment 2
[0061] Synthesis of squaraine dye SQ-7 (1,3 bis((1-methyl-2,3,3 trimethylindoline)-methylene) squaraine
[0062] (1) Synthesis of 2,3,3-trimethylindoline
[0063] Add 10ml of phenylhydrazine and 11ml of methyl isopropyl ketone, 150ml of absolute ethanol and 10ml of concentrated sulfuric acid in a 250ml three-necked flask, stir and reflux for 10 hours under nitrogen protection, after removing ethanol, the residue is neutralized with sodium carbonate solution, Separate the organic layer, extract the aqueous layer three times with 30ml ether, combine with the organic layer, dry over anhydrous magnesium sulfate, remove the solvent by rotary evaporation, and distill under reduced pressure under the protection of nitrogen to collect the fraction at 88-92°C / 3mmHg. Yield 50%.
[0064] (2) Synthesis of 2,3,3-trimethylindoline methyl iodide salt
[0065] Add 4g of 2,3,3-trimethylindoline, 4ml of methyl iodide and 10ml of chloroform into a 100ml three-necked flask, stir and reflux for ...
Embodiment 3
[0069] Synthesis of squaraine dye SQ-8 (1,3 bis((1-methyl-benzothiazolyl)-methylene) squaraine
[0070] (1) Synthesis of N-methylbenzothiazole iodine salt
[0071] In a 100ml round bottom flask, add 12.7ml of benzothiazole, 12.5ml of iodomethane, 20ml of ethanol, install a condenser, stir in an oil bath, and reflux for 7 hours (oil bath temperature 65-70°C). The reaction was stopped and cooled to room temperature, filtered and dried to obtain 12.5 g of white powder with a yield of 45%.
[0072] (2) Synthesis of SQ-8
[0073] In a 250ml round bottom flask, add 1.0g of squaraine, 6g of N-methylbenzothiazole iodine salt, 96ml of n-butanol, 24ml of benzene, 3ml of quinoline, install a water separator, and put it on the water separator to condense Tube, oil bath, stirring, reflux (oil bath temperature 110-120° C.), and maintain the reaction for 15 hours. After the reaction was stopped, it was cooled to room temperature, left to stand overnight and then filtered. The product was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com