Antimicrobial peptide Ea-CATH1 of Equus asinus and genes and application thereof
An antimicrobial peptide, ea-cath1 technology, applied in the field of biomedicine, can solve the problems that the research on active protein peptides has not yet been reported, and achieve the effect of strong killing effect, small molecular weight, and rapid bactericidal action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Cloning and gene sequencing of donkey antimicrobial peptide Ea-CATH1 precursor gene, including:
[0030] The total RNA of donkey spleen was extracted with RNeasy Mini Kit, and the Reverse Transcriptase reverse transcription synthesis cDNA first strand. Two specific forward primers (P1, P2) and one reverse non-specific universal primer (CDSIII) were designed, and the cDNA of donkey cathelicidin gene was amplified by semi-nested PCR using single-stranded cDNA as a template.
[0031] Forward P1: 5′-GGACCATGGAGACCCAGAGG-3′;
[0032] Forward P2: 5′-ATGGAGACCCAGAGGGACAGTT-3′;
[0033] Reverse Non-specific Universal Primer for CLONTECH Creator TM SMART TM 3'PCR Primer CDSIII in the cDNA LibraryConstruction Kit, its sequence is: 5'-ATTCTAGAGGCCGAGGCGGCCGACATGT(30)N -1 N-3' (N=A, G, C, or T; N -1 = A, G, or C).
[0034] The obtained positive single clones were subjected to gene nucleotide sequence determination, and the general primers for pMD19-T vector sequencing:
...
Embodiment 2
[0084] The chemical synthesis method of Ea-CATH1:
[0085] 1. According to the amino acid sequence of mature peptide Ea-CATH1 deduced from the gene encoding domestic animal donkey cathelicidin antimicrobial peptide, its full sequence was synthesized with an automatic peptide synthesizer (433A, Applied Biosystems), and purified by HPLC reverse-phase column chromatography desalting.
[0086] II. Molecular weight was determined by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF).
Embodiment 3
[0088] Pharmacological experiment of donkey antimicrobial peptide Ea-CATH1 from livestock:
[0089] 1. Detection of antibacterial activity of Ea-CATH1:
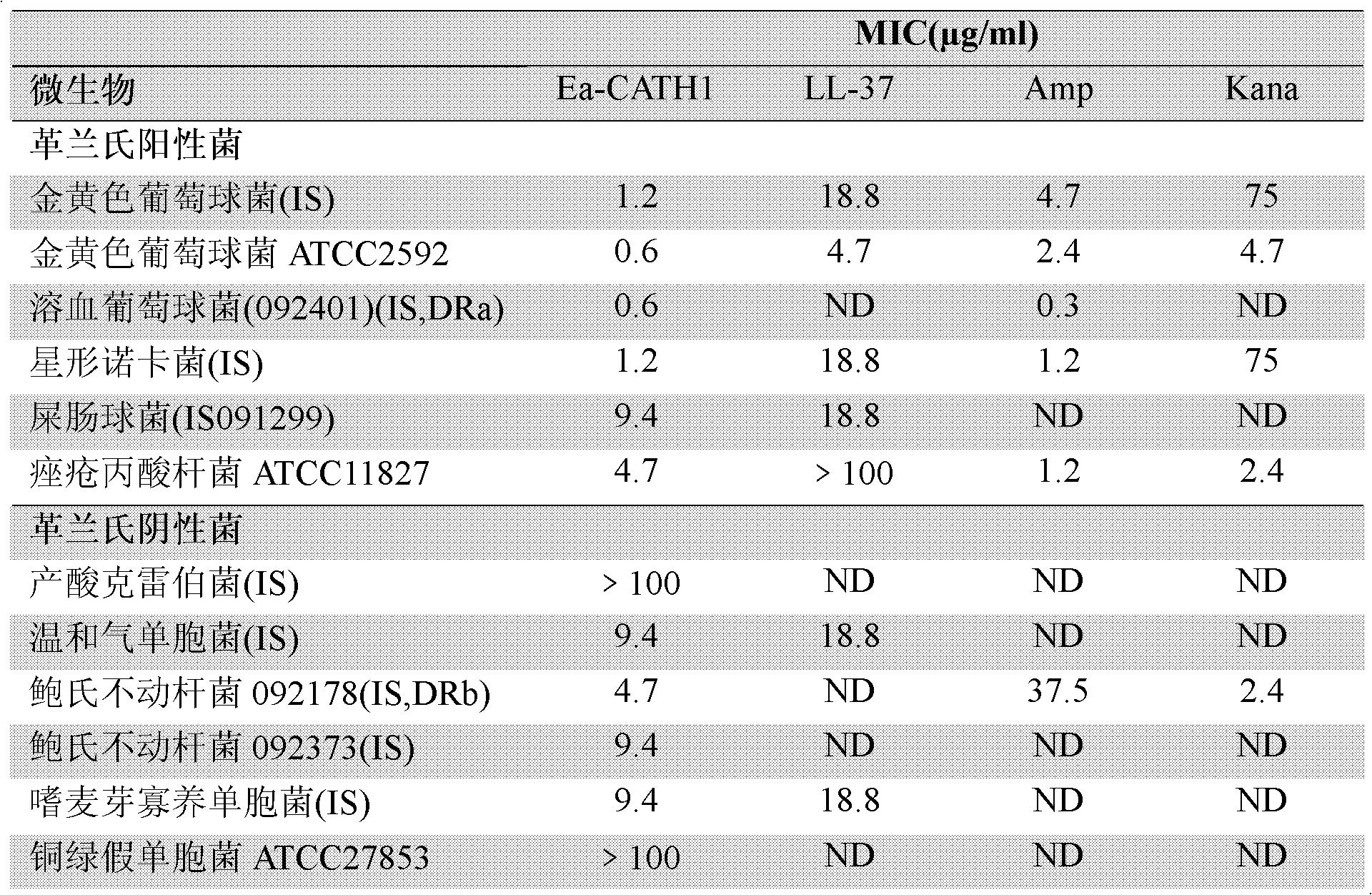
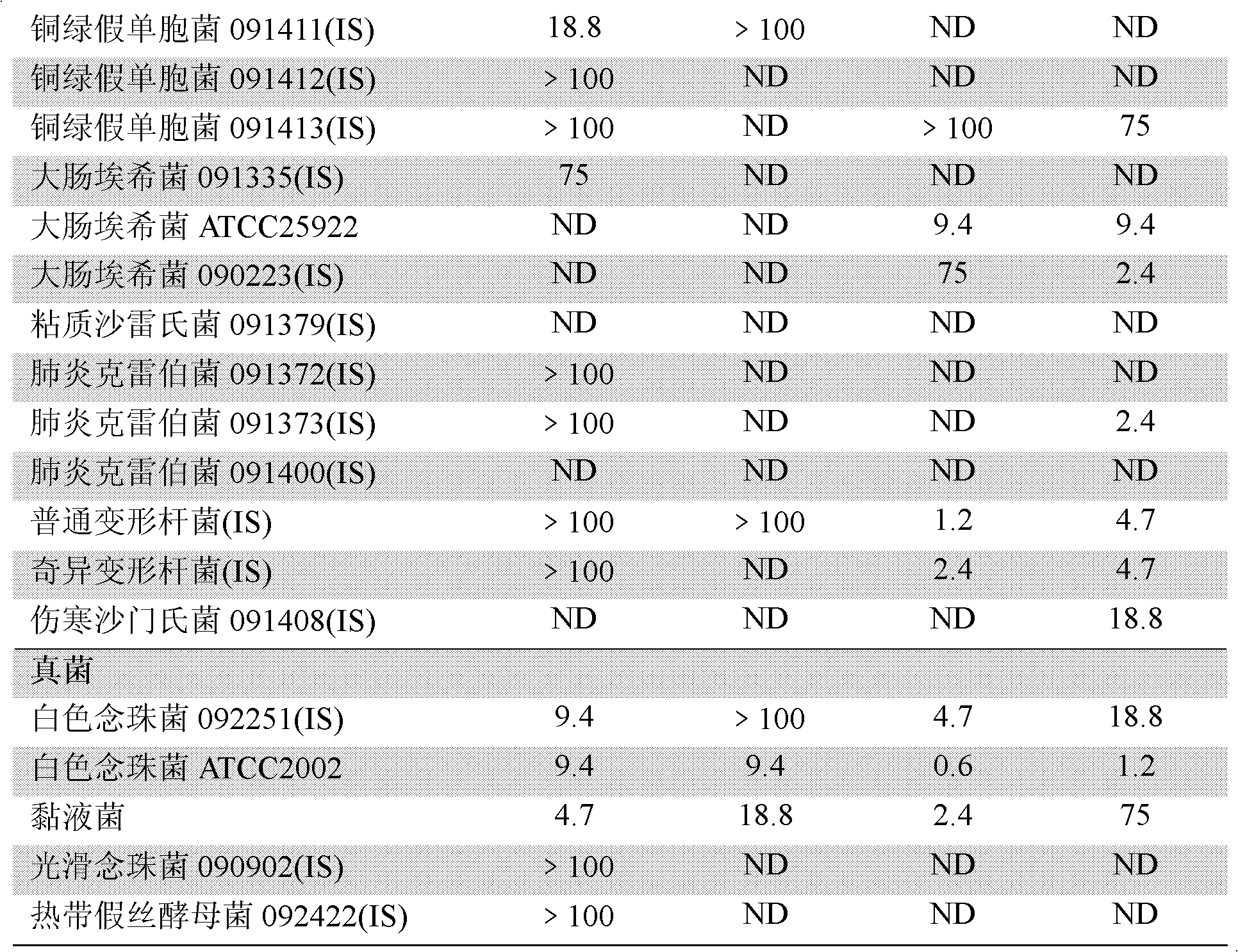
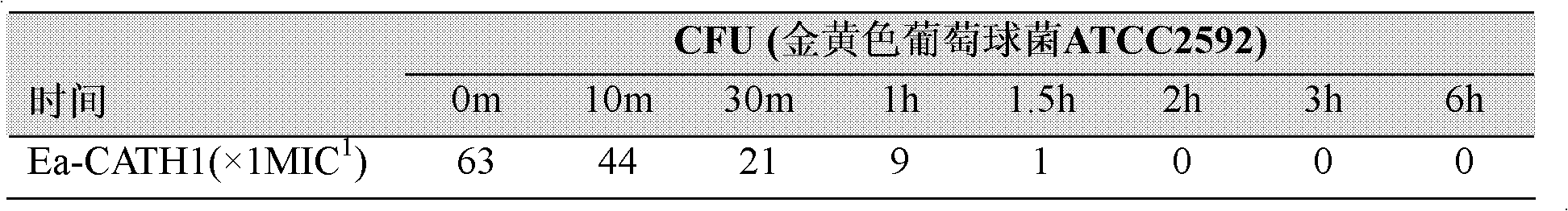
[0090] Dissolve chemically synthesized Ea-CATH1 in sterile ultrapure water at a concentration of 2 mg / ml; pick up newly activated microorganisms with an inoculation loop, and spread them evenly on a new LB agar plate; place a circle with a diameter of 0.5 cm Place a piece of sterile filter paper on the above-mentioned agar plate, and then drop 10 μl of Ea-CATH sample solution onto the piece of paper; put it in a constant temperature incubator at 37°C for 12-24 hours; CATH1-sensitive strains were recorded.
[0091] 2. Determination of Ea-CATH1 on the minimum inhibitory concentration (Minimum Inhibitory Concentration, MIC) of sensitive strains. In this experiment, human cathelicidin LL-37, traditional antibiotics ampicillin and kanamycin were used as positive controls, and sterile liquid LB was used as negative controls; the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com