Cross-linkable fluorobenzene-containing end-capped conjugated polymer based on benzodithiophene and double thiophene-substituted difluorobenzothiadiazole and application thereof to solar cell
A technology of difluorobenzothiadiazole and conjugated polymers, which is applied in circuits, photovoltaic power generation, electrical components, etc., to achieve the effects of improving stability and service life, improving hole and electron transport rates, and promoting uniform dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
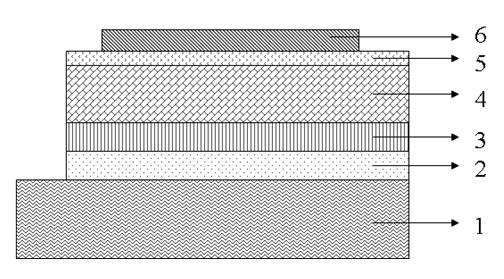
Image
Examples
Embodiment 1
[0037] Example 1: Pentafluorophenyl-terminated poly[4,8-bis(7-octenyloxy)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl -5,6-difluoro-4,7-bis(2-thienyl)-2,1,3-benzothiadiazole] alternating copolymer, the implementation steps are as follows.
[0038] Add 4,8-dihydrobenzo[1,2-b:4,5-b']dithiophene-4,8-diketone (2.3 g, 10 mmol), zinc powder (1.5 g, 23 mmol) 35 mL of water, then add 6.4 g of NaOH, stir and heat under reflux for 4 h. Then 8-bromooct-1-ene (6.1 g, 32 mmol) and a small amount of tetrabutylammonium bromide were added to the reaction system and refluxed for 8 h. After the reaction, the reactant was poured into cold water, extracted with ether, and the organic phase was extracted with anhydrous MgSO 4 Concentrate after drying, and then purify with silica gel column to obtain the product 1, [4,8-bis(7-octenyloxy)benzo[1,2-b:4,5-b']dithiophene ].
[0039]
[0040] Under nitrogen atmosphere, 4,8-bis(7-octenyloxy)benzo[1,2-b:4,5-b']dithiophene (0.31 g, 0.9 mmol) was dissolve...
Embodiment 2
[0045] Example 2: Pentafluorophenyl-terminated poly[4,8-bis(6-bromohexyloxy)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl The preparation of -5,6-difluoro-4,7-bis(2-thienyl)-2,1,3-benzothiadiazole] alternating copolymer is similar to Example 1, and the implementation steps are as follows.
[0046]
[0047] Add 4,8-dihydrobenzo[1,2-b:4,5-b']dithiophene-4,8-diketone (2.3 g, 10 mmol), zinc powder (1.5 g, 23 mmol) 35 mL of water, then add 6.4 g of NaOH, stir and heat under reflux for 4 h. Then 1,6-dibromo-n-hexane (7.8 g, 32 mmol) and a small amount of tetrabutylammonium bromide were added to the reaction system and refluxed for 8 h. After the reaction, the reactant was poured into cold water, extracted with ether, and the organic phase was extracted with anhydrous MgSO 4 Concentrate after drying, and then purify with silica gel column to obtain 4,8-bis(6-bromohexyloxy)benzo[1,2-b:4,5-b']dithiophene.
[0048] Under nitrogen atmosphere, 4,8-bis(6-bromohexyloxy)benzo[1,2-b:4,5-b']di...
Embodiment 3
[0051] Example 3: Preparation of a polymer solar cell device.
[0052] 10 mg of pentafluorophenyl-terminated poly[4,8-bis(7-octenyloxy)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl-5 ,6-difluoro-4,7-bis(2-thienyl)-2,1,3-benzothiadiazole] mixed with 30 mg PCBM, added 2 mL of chlorobenzene solution, and sprayed on PEDOT by spin coating : A thin film is prepared on the ITO glass modified by PSS, and then the cathode is prepared by vacuum evaporation of lithium fluoride and aluminum.
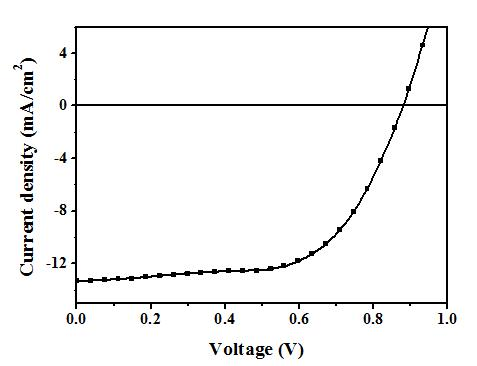
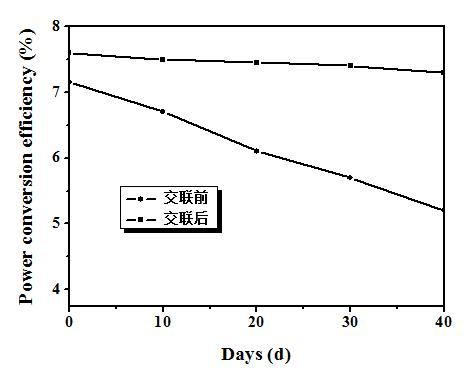
[0053] The device performance is: standard simulated sunlight (AM 1.5 G, 100 mW / cm 2 ) under irradiation, open circuit voltage = 0.88 V; short circuit current = 13.33 mA / cm 2 ; Fill factor = 65%; Energy conversion efficiency = 7.6%. Its current-voltage curve is attached as figure 2 As shown, the performance stability of the device before and after crosslinking is as follows image 3 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com