Dual-functional oleanolic acid derivatives as well as preparation method and application thereof
An oleanolic acid and dual-function technology, which is applied in the field of oleanolic acid derivatives and their preparation, can solve the problems of excessive inhibition of bone turnover, the need for subcutaneous administration, and inaccurate long-term efficacy, and achieves inhibition of osteoclasts. Cell production and bone resorption activity, bone formation promotion activity, platelet aggregation inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
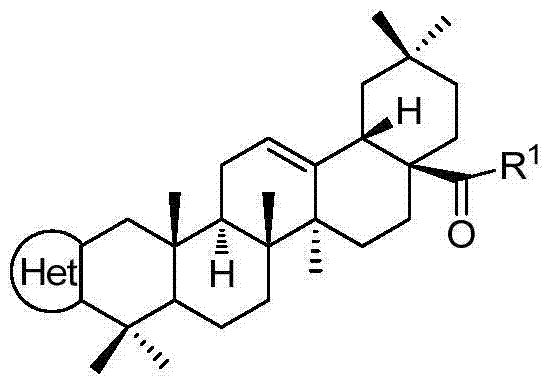
Embodiment 1
[0029] 1. The preparation of compound 1, the reaction formula is:
[0030]
[0031] Preparation of Compound A
[0032] Na 2 HPO 4 12H 2 O (1.7g, 4.8mmol) and NaH 2 PO 4 2H 2 O (0.75g, 4.8mmol) was fully dissolved in 95mL of water to prepare a phosphate buffer solution. Na 2 WO 4 2H 2 O (4g, 12mmol) dissolved in 35% aqH 2 o 2 (28mL) and added to phosphate buffered saline. Add OA (11g, 24mmol) and DMF (50mL) in a 500mL round bottom flask, and heat to 90°C, add the above Na 2 WO 4 -H 2 o 2 Phosphate buffered saline, and the reaction was stirred for 1 hour. After the reaction was completed, it was cooled to room temperature, extracted with ethyl acetate (100 mL×3), the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain compound A (9.8 g, 89%).
[0033] Preparation of Compound B
[0034] A 250 mL round bottom flask was charged with compound A (9.0 g, 20 mmol), toluene (100 mL). Sodium methoxide (2.8 g, 50 mm...
Embodiment 2
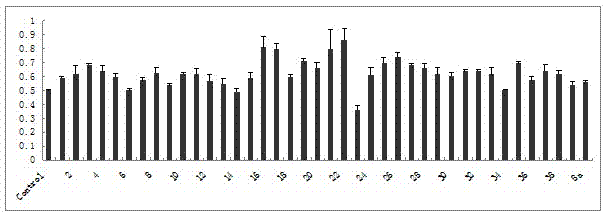
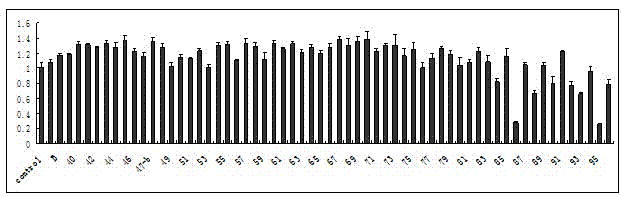
[0304] Example 2 Specific activity examples of oleanolic acid derivatives:
[0305] 1 Inhibition of osteoclasts generated in vitro
[0306] Osteoclast induction in vitro: 1) RAW264.7 cells were passaged 2-3 times with a disposable cell scraper after recovery, and the confluence was 60-70% (the differentiation potential of RAW264.7 cells was significantly reduced after being completely overgrown and subcultured), and the cells were in Scrape off with a cell scraper during logarithmic division, centrifuge at 1000g for 10min, resuspend in phenol red-free α-MEM containing 10% heat-inactivated FBS and 30ng / mL RANKL, count, and use 1X10 4 mL -1 The density was seeded in a 96-well cell culture plate with 100 μ cell solution per well. 2) Replace half of the phenol red-free α-MEM medium containing 10% heat-inactivated FBS and 30ng / mL RANKL every other day. 3) TRAP staining was performed on the second day of culture, and more than 90% of the cell populations were TRAP-positive mononu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com