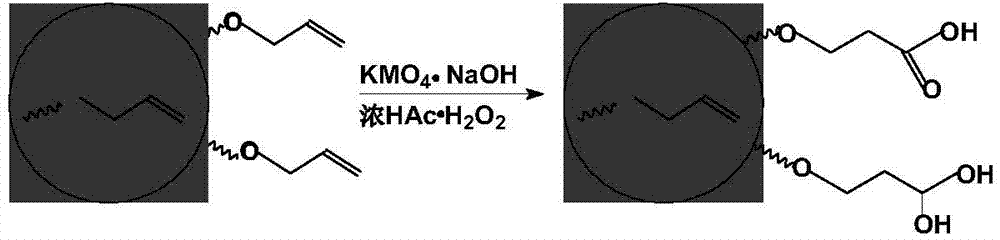
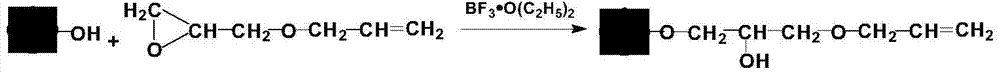Method for preparing fibrinogen by expanded bed adsorption (EBA) technique
A technology of fibrinogen and fibrin, which is applied in the direction of preparation methods of fibrinogen and peptides, chemical instruments and methods, etc., can solve problems such as no change, achieve reduction of chemical substances, improvement of process continuity and automation, The effect of improving safety and quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of ion exchange chromatography medium for expanded bed (EBA)
[0049] 1. Preparation of cross-linked agarose-tungsten carbide composite beads
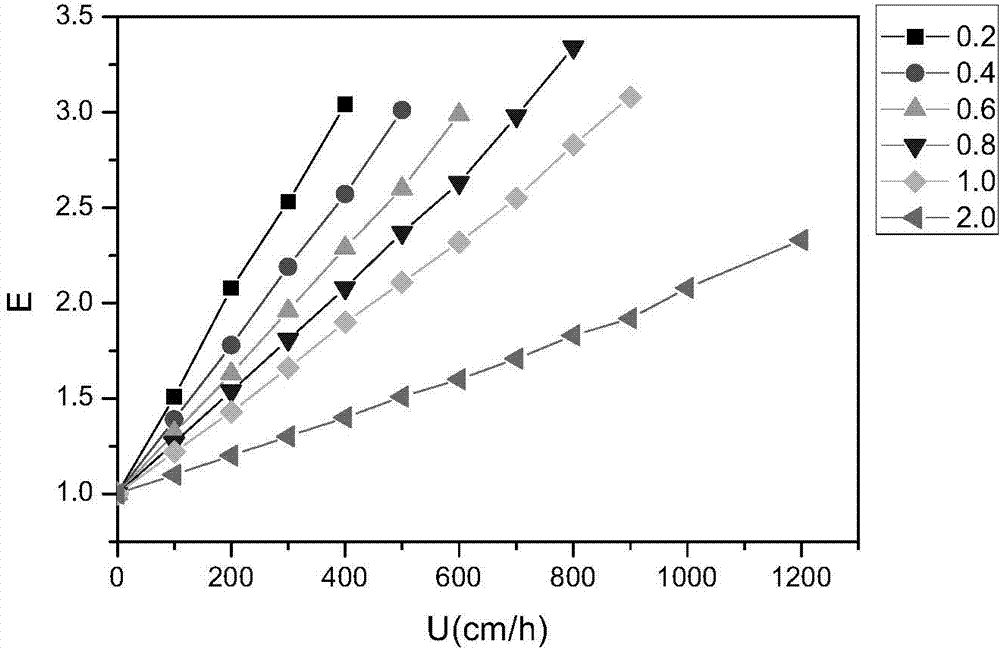
[0050] Take 2 g of drained agarose gel and 0.5 g of NaCl to make 4% (W / W) agarose-water suspension. According to the traditional method of thermal regeneration of reverse phase suspension, 50g of 4% agarose-water suspension was transferred to the flask, stirred and mixed at room temperature for 15 minutes, and a series of different amounts of tungsten carbide (xlwc100, Chaozhou Xianglu Tungsten Co., Ltd., China, average particle size 9-11μm), add tungsten carbide and agarose gel at mass ratios of 0.2, 0.4, 0.6, 0.8, 1 and 2, stir the mixture at 600rpm for 15min, heat to 93°C for 60min, as the water phase spare. Then, 200mL of paraffin and petroleum ether (10:1, V / V), 4% emulsifier (Span80:Tween80=10:4) mixture was ultrasonicated for 5-10min, and preheated in a 93°C water bath as the oil phase for later us...
Embodiment 2
[0080] The liquid preparation of embodiment 2 EBA chromatography, the treatment of medium and packing column
[0081] Chromatographic medium: Agarose (tungsten carbide)-DEAE was synthesized according to the method in Example 1; XP 2*60 chromatography column, STREAMLINE 100, 10*100 were purchased from GE Company.
[0082] Chromatography buffer: A balance solution (40mM sodium citrate pH5.0), B eluent (10mM sodium citrate pH5.0), B1 eluent 1 (B+5g / L sodium octanoate / HCl pH6. 0), B2 eluent 2 (1M sodium citrate pH8.0+0.3M sodium chloride pH8.0), B3 eluent 3 (20mM sodium citrate+0.1M sodium chloride pH8.0), C regeneration Solution (1M NaOH) was prepared according to routine.
[0083] Rinse the agarose (tungsten carbide)-DEAE medium with 1:1 (V / V) double distilled water. When the medium is completely precipitated and separated from water, pour the supernatant and repeat three times. Evacuate the air bubbles through the bottom of the column, add water to soak the bottom surface, an...
Embodiment 3
[0084] Example 3 Expanded bed action column chromatography (EBA) separation of raw plasma (column bed 25cm high)
[0085] Column equilibration (A balance solution 2.5CV) → plasma preparation (dilution × 3, adjusted to pH 5.0 with HCl) → sample loading (injection from the lower end of the column) (1.5L plasma / L adsorbent amount) → non-adsorbing Plasma fraction and eluate (2.5CV)→elution fraction 1 (1CV)→elution fraction 2 (1CV)→elution fraction 3 (1CV)→CIP (1CV) / equilibrium / first elution (Ultraviolet spectrophotometer adjusted to 280nm)
[0086] XP 2*60 column was used in the experiment (column bed 25cm high), one chromatography was performed with a linear velocity of 5cm / min, and one chromatography was performed with a linear velocity of 10cm / min. Dilute the raw plasma (liquid state) three times and load it directly. According to the amount of 1.5L plasma / L adsorbent (diluted and adjusted pH) / sample, the experimental results of the two chromatography are shown in Table 3.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com