Triad fullerene derivative and preparation method and application thereof
A fullerene derivative and fullerene technology, applied in the preparation of organic compounds, carboxylic acid nitrile preparation, chemical instruments and methods, etc., can solve the problem of curbing the development of organic polymer heterojunction solar cells and restricting the development of fullerenes The commercial application of derivatives and the lack of research on new fullerene derivative acceptor materials have achieved the effects of enhancing self-assembly performance, improving photoelectric conversion efficiency, and increasing LUMO energy level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
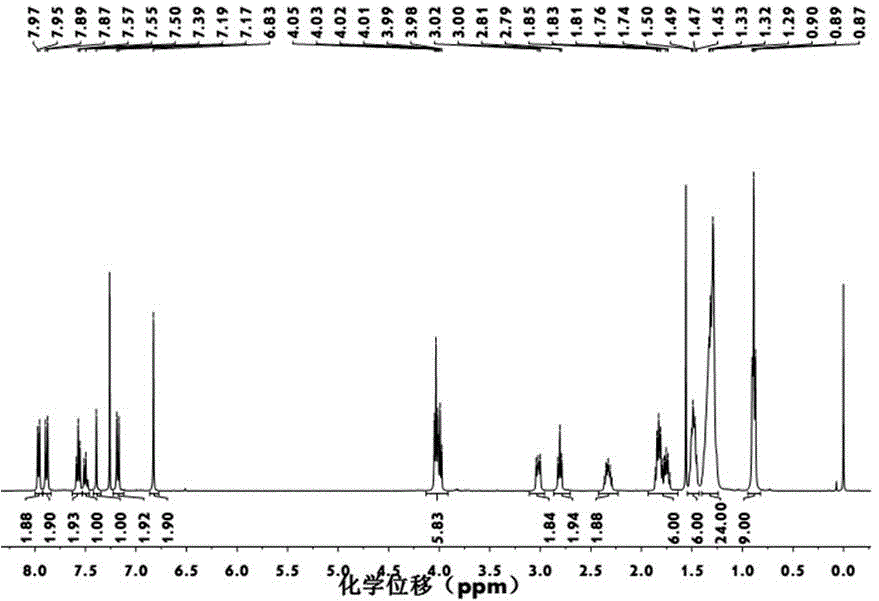
[0068] A method for preparing a triplet fullerene derivative with cooperative assembly performance, specifically comprising the following steps:
[0069] (1) Phosphorus tribromide (0.40 g, 1.48 mmol) in CH 2 Cl 2 (2 mL) solution was added to 3,4,5-trioctyloxybenzyl alcohol (0.34 g, 0.70 mmol) in CH under ice-cooling 2 Cl 2 (4 mL) reaction in the solution, stirred at room temperature for 3h to obtain 3,4,5-trioctyloxybenzyl bromide;
[0070] (2) In acetonitrile (10 mL) solution, 3,4,5-trioctyloxybenzyl bromide (0.24 g, 0.43 mmol) and trimethylsilyl cyanide (90 μL) were dissolved in tetrabutylammonium fluoride (700 μL) was activated at room temperature for 2.5h to obtain 3,4,5-trioctyloxycyanide;
[0071] (3) Under argon protection, a solution of sodium methoxide (0.03 g, 0.63 mmol) in ethanol (2 ml) was added dropwise to 3,4,5-trioctyloxycyanide (0.11 g, 0.21 mmol) under reflux conditions React with p-hydroxybenzaldehyde (0.03 g, 0.21 mmol) in ethanol (4 mL) for 12 hour...
Embodiment 2
[0084] According to the preparation steps of Example 1, the carbon chain length of the alkoxy group of the substituted benzyl alcohol compound can be changed to 4-20, and can be replaced by other flexible functional groups at the same time, which can be used to replace the phenolic hydroxyl group in p-hydroxybenzaldehyde. Para-position introduces electron-withdrawing groups such as nitro, cyano, etc., using C 70 、C 84 etc instead of C 60 ; Triad fullerene derivatives with various structures can be obtained.
[0085] For example, replace 3,4,5-trioctyloxybenzyl alcohol with 3,4,5-tris(2-(2-(2-methoxyethoxy)ethoxy)ethoxybenzyl alcohol, and p-Hydroxybenzaldehyde is replaced by 3-nitro4-hydroxybenzaldehyde; the reaction results in a three-body fullerene derivative, referred to as PCBB-NO 2 -CN-6C-EG, the structural formula is:
[0086]
[0087] Include the following steps:
[0088] (1) Phosphorus tribromide (0.38 g, 1.48 mmol) in CH 2 Cl 2 (2 mL) solution was added to ...
Embodiment 3
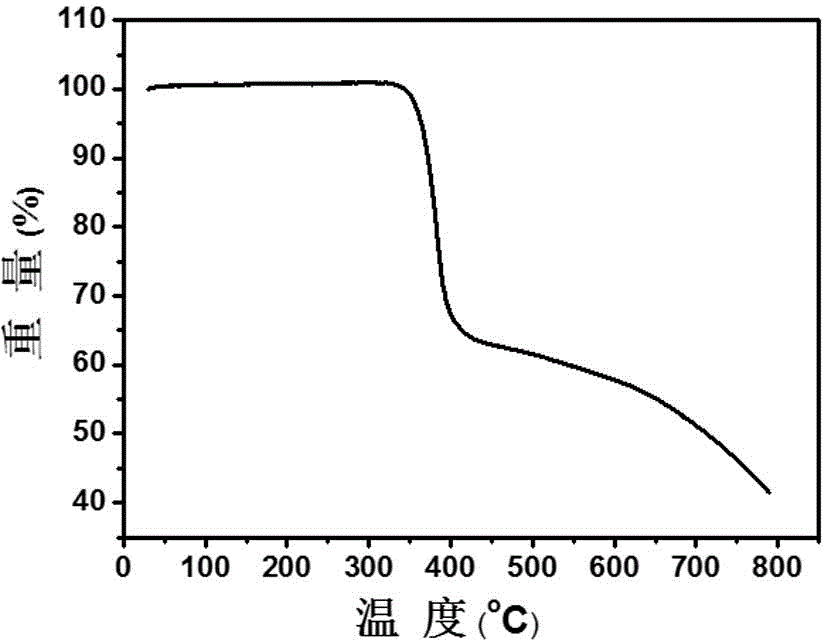
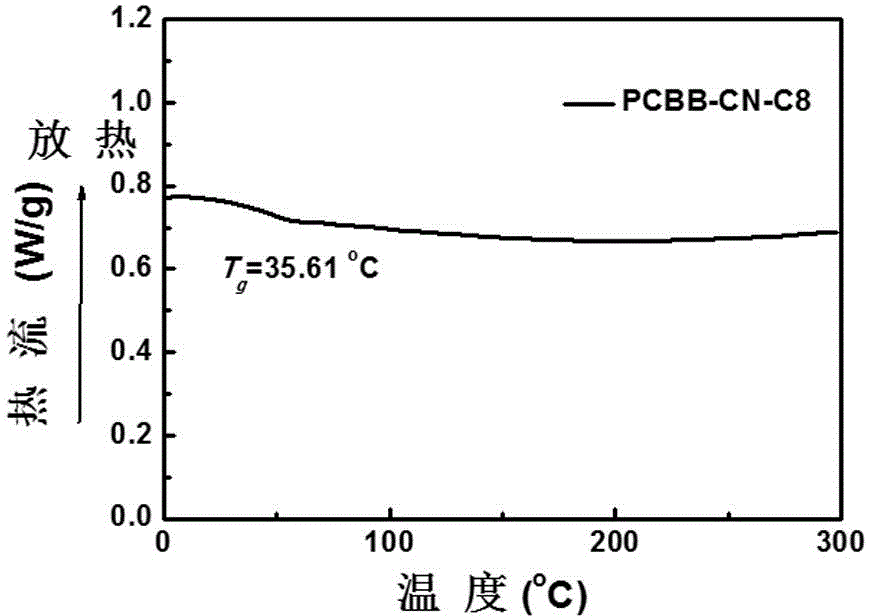
[0095] An active layer material for an organic polymer solar cell, comprising the above-mentioned three-body fullerene derivative and a donor material. Taking PCBB-CN-C8 as the acceptor material and P3HT as the donor material:
[0096] (1) Use ethanol, acetone, and isopropanol to ultrasonically dry the glass sheet, spin-coat a PEDOT:PSS layer at a speed of 3000 rpm as the anode modification layer (electron blocking layer), and then heat and dry at 150°C for 10 minutes. remove moisture;
[0097] (2) Use o-dichlorobenzene (ODCB) as a good solvent to prepare a mixed solution of P3HT / PCBB-CN-C8 (w / w 1 / 1.25), stir it, and then spin-coat it on the anode modification at a speed of 1000rpm layer; unannealed film was obtained;
[0098] (3) Take one piece of P3HT / PCBB-CN-C8 in the glove box and anneal at 100°C for 10 minutes. The resulting unannealed and annealed films can be used to test the transmission electron microscope, selected area electron diffraction pattern (see Figure 7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com