Supported nickel-copper alloy nano-catalyst and preparation method thereof and application to catalytic hydrogenation
A nano-catalyst, nickel-copper alloy technology, applied in the direction of metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, preparation of organic compounds, etc., can solve the problem of low purity and crystallinity of supported catalysts, metal and Weak carrier interaction, complex catalyst preparation process and other issues, to achieve the effect of green production process, high catalytic activity and stability, simple magnetic separation and recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
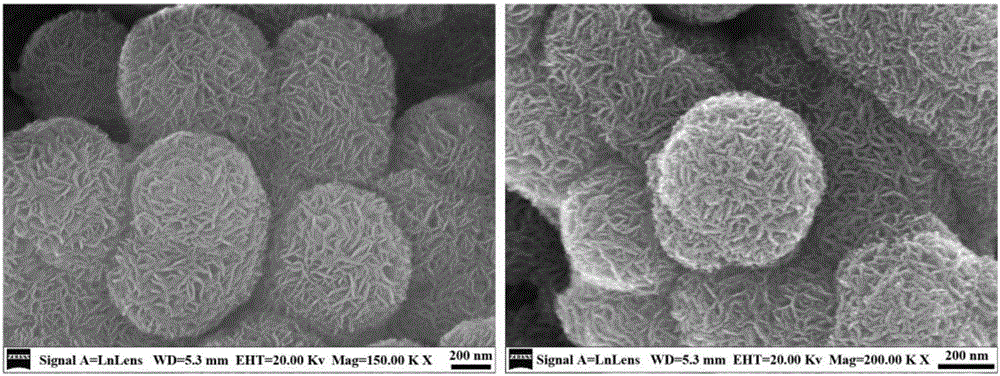
[0029] 1) Make 50mL concentration 0.15mol L -1 Nickel nitrate, 50mL concentration is 0.05mol L -1 Copper nitrate aqueous solution and 50mL concentration is 0.4mol L -1 The aqueous solution of sodium salicylate was evenly mixed, and after stirring for 10 minutes, slowly added 50 mL of -1 The urea aqueous solution was reacted in a nitrogen atmosphere at 95°C for 36 hours. The product was centrifuged, washed and dried to obtain a layered nickel hydroxide precursor with a large amount of organic anion intercalation. The scanning electron microscope photo is shown in figure 2 ;
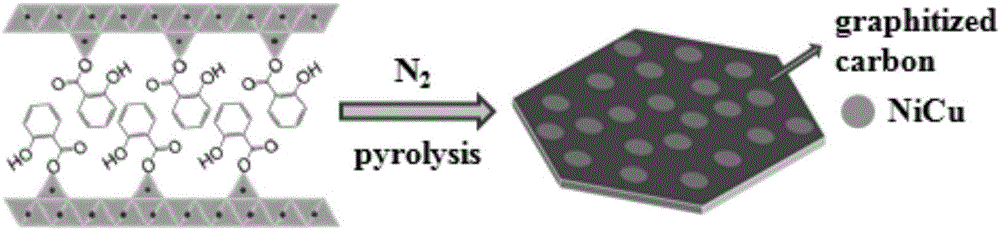
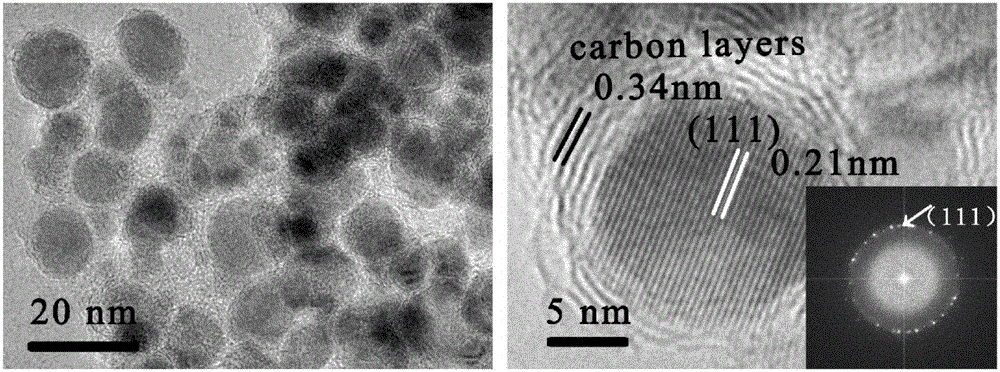
[0030] 2) The precursor prepared in the above steps was calcined at 500°C for 1 hour in a nitrogen atmosphere to obtain a nickel-copper alloy / carbon nanocomposite catalyst, which was denoted as NiCu / C. For the characterization results, see Figure 2-6 , the product was used to evaluate the catalytic hydrogenation performance of p-nitrophenol.
Embodiment 2
[0032] 1) Make 50mL concentration 0.12mol L -1 Nickel nitrate, 50mL concentration is 0.08mol L -1 Copper nitrate aqueous solution and 50mL concentration is 0.4mol L -1 The aqueous solution of sodium salicylate was evenly mixed, and after stirring for 10 minutes, slowly added 50 mL of -1 The urea aqueous solution was reacted in an argon atmosphere at 95°C for 36 hours, and the product was centrifuged, washed and dried to obtain a layered nickel hydroxide precursor with a large amount of organic anion intercalation;
[0033] 2) The precursor prepared in the above steps was calcined at 700°C for 2 hours in a nitrogen atmosphere to obtain a nickel-copper alloy / carbon nanocomposite catalyst, denoted as NiCu / C-1, and the characterization results are as follows Figure 4 with Figure 5 , the product was used to evaluate the catalytic hydrogenation performance of p-nitrophenol.
Embodiment 3
[0035] 1) Make 50mL concentration 0.1mol·L -1 Nickel nitrate, 50mL concentration is 0.1mol L -1 Copper nitrate aqueous solution and 50mL concentration is 0.4mol L -1 The aqueous solution of sodium salicylate was evenly mixed, and after stirring for 10 minutes, slowly added 50 mL of -1 The urea aqueous solution was reacted in a nitrogen atmosphere at 95°C for 48 hours, and the product was centrifuged, washed and dried to obtain a layered nickel hydroxide precursor with a large amount of organic anion intercalation;
[0036] 2) The precursor prepared in the above steps was calcined at 600°C for 1 hour in a nitrogen atmosphere to obtain a nickel-copper alloy / carbon nanocomposite catalyst, which was denoted as NiCu / C-2. For the characterization results, see Figure 4 with Figure 5 , the product was used to evaluate the catalytic hydrogenation performance of p-nitrophenol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com