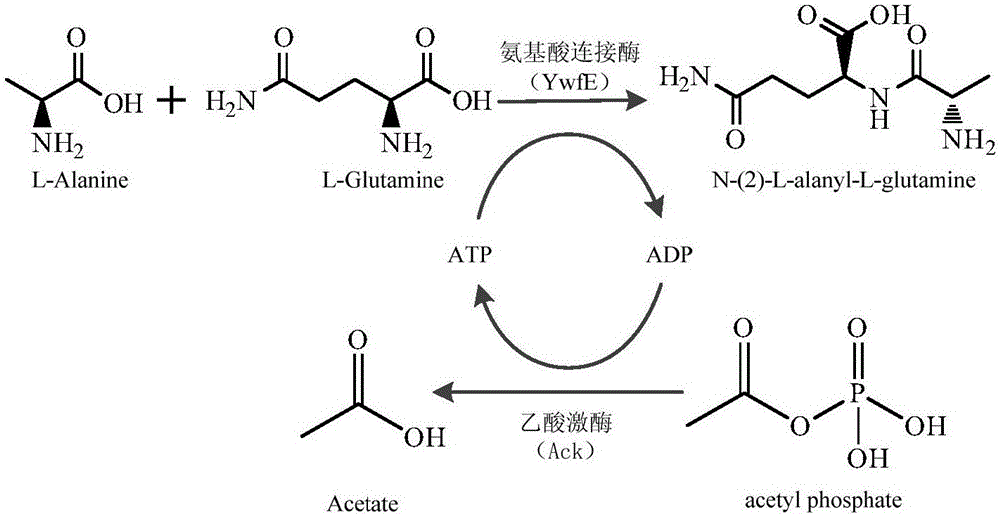
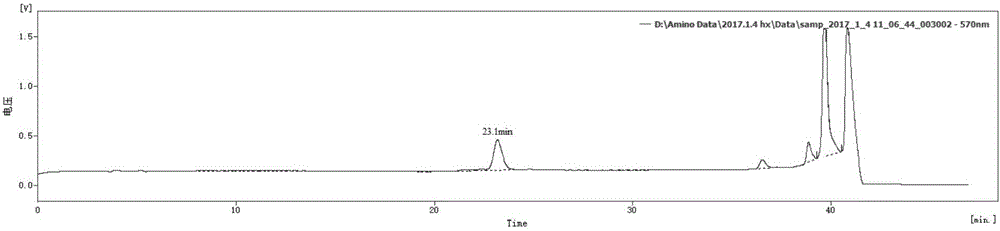
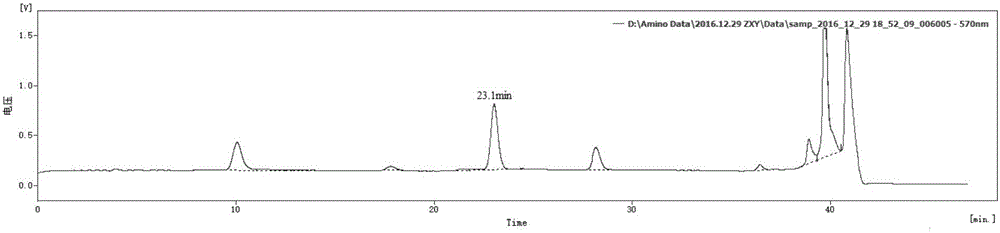
Preparation method of glutamine dipeptide
A glutamate dipeptide and alanine technology, which is applied in biochemical equipment and methods, peptides, enzymes, etc., can solve the problems of complex synthesis process, unfavorable industrial scale-up, high production cost, and achieve wide source of raw materials, easy large-scale industrial production, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The construction of embodiment 1 amino acid ligase bacterial strain
[0024] ①According to the nucleotide sequence of the amino acid ligase encoding gene ywfE of B. subtilis ATCC 15245 on Genbank, use the codon software commonly used in Escherichia coli to optimize its codon, and add the restriction site BamH I to the optimized sequence and Hind III (sequence shown in Sequence Listing 400) were sent to Jinweizhi Company for synthesis.
[0025] ② Use Takara restriction endonucleases BamH I and Hind III to double digest the target gene fragment and pET-His vector plasmid in step ① to obtain ywfE and pET-His linear fragments with the same cohesive ends.
[0026] ③ Use Takara T4 DNA ligase to connect the two gene fragments in step ② to obtain the recombinant expression vector pET-His-ywfE.
[0027] ④ Transform the recombinant expression vector in step ③ into E.coli BL21 (ACCC11171) to obtain the amino acid ligase-producing strain E.coli pET-His-ywfE.
Embodiment 2
[0028] The construction of embodiment 2 acetate kinase bacterial strains
[0029] ①A pair of gene amplification primers ( The upstream primer is the sequence shown in Sequence Listing 400 , the downstream primer is the sequence shown in Sequence Listing 400 ), and the ack gene fragment is obtained by amplification. The pair of primers respectively comprise enzyme cutting sites BamH I and EcoRI.
[0030] ② Use Takara restriction endonucleases BamH I and EcoRI to double digest step ① to obtain the target fragment and pET-His vector plasmid, and obtain ack and pET-His linear fragments with the same sticky ends.
[0031] ③ Use Takara T4 DNA ligase to connect the two gene fragments obtained in step ② to obtain the recombinant expression vector pET-His-ack.
[0032] ④ Transform the recombinant expression vector in step ③ into E.coli BL21 (ACCC11171) to obtain the acetate kinase-producing strain E.coli pET-His-ack.
Embodiment 3
[0033] The preparation of embodiment 3 amino acid ligase and acetate kinase
[0034] ① Inoculate the strains in (1) and (2) from the glycerol bacteria preservation tube with an inoculum of 0.1% (v / v) into 100 mL of LB liquid medium containing ampicillin (100 μg / mL), and culture at 37°C and 200 rpm After 12 hours, transfer to 400 mL LB liquid medium containing ampicillin (100 μg / mL) according to 1% (v / v) inoculum amount, and continue culturing at 37° C. and 200 rpm. Strain concentration OD 600nm When it reached 0.6, IPTG with a final concentration of 0.1 mmol / L was added, and the expression protein was induced and cultured at 28°C for 10 h.
[0035] ② After the cultivation, the fermentation broth was centrifuged at 4°C and 6000rpm to collect the bacterial cells. After washing three times with PBS buffer at pH 7.4, resuspend with PBS buffer at pH 7.4, sonicate for 20 min, and centrifuge at 4°C at 10,000 rpm to obtain the supernatant.
[0036] ③ Pass the supernatant through Ni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com