Application of berberine ion pair compound to preparation of anti-breast-cancer drug
A technology of compound and ion pair, which is applied in the field of medicine, can solve the problems of toxic side effects, low oral availability, high price, etc., and achieve the effects of small toxic side effects, obvious curative effect, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 The in vitro inhibitory effect of braidin on triple-negative breast cancer MDA-MB-231 cells
[0045] 1.1 Cell culture MCF-7 and MDA-MB-231 cells were respectively used in DMEM and L-15 medium, containing fetal bovine serum with a volume fraction of 0.1, 100 K U·L -1 Penicillin and 100 mg·L -1 Streptomycin, set at 37°C, volume fraction 0.05 and CO 2 , Routine culture in an incubator with saturated humidity, digested and passaged with 0.25% trypsin. Cells in the exponential proliferation phase 3 days after subculture were used in the experiment;
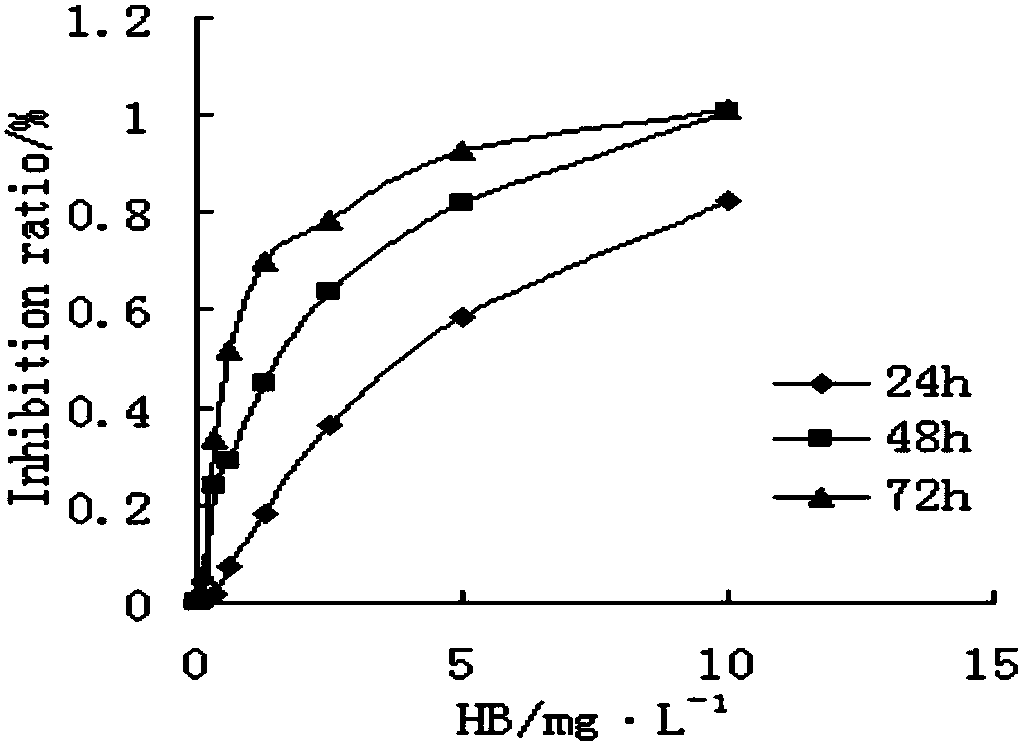
[0046] 1.2 Method to detect the effect of huanggui on the proliferation of breast cancer cells Collect tumor cells in logarithmic growth phase, prepare single cell suspension with complete culture medium, inoculate in 96-well plate, MCF-7 cells per well 8×10 3 1.2×10 MDA-MB-231 cells per well 4 set CO 2 incubator overnight. Set up vehicle control group and different concentrations of bradycardin treatment groups...
Embodiment 2
[0052] Example 2 The effect of huanggui on the MDA-MB-231 cell cycle
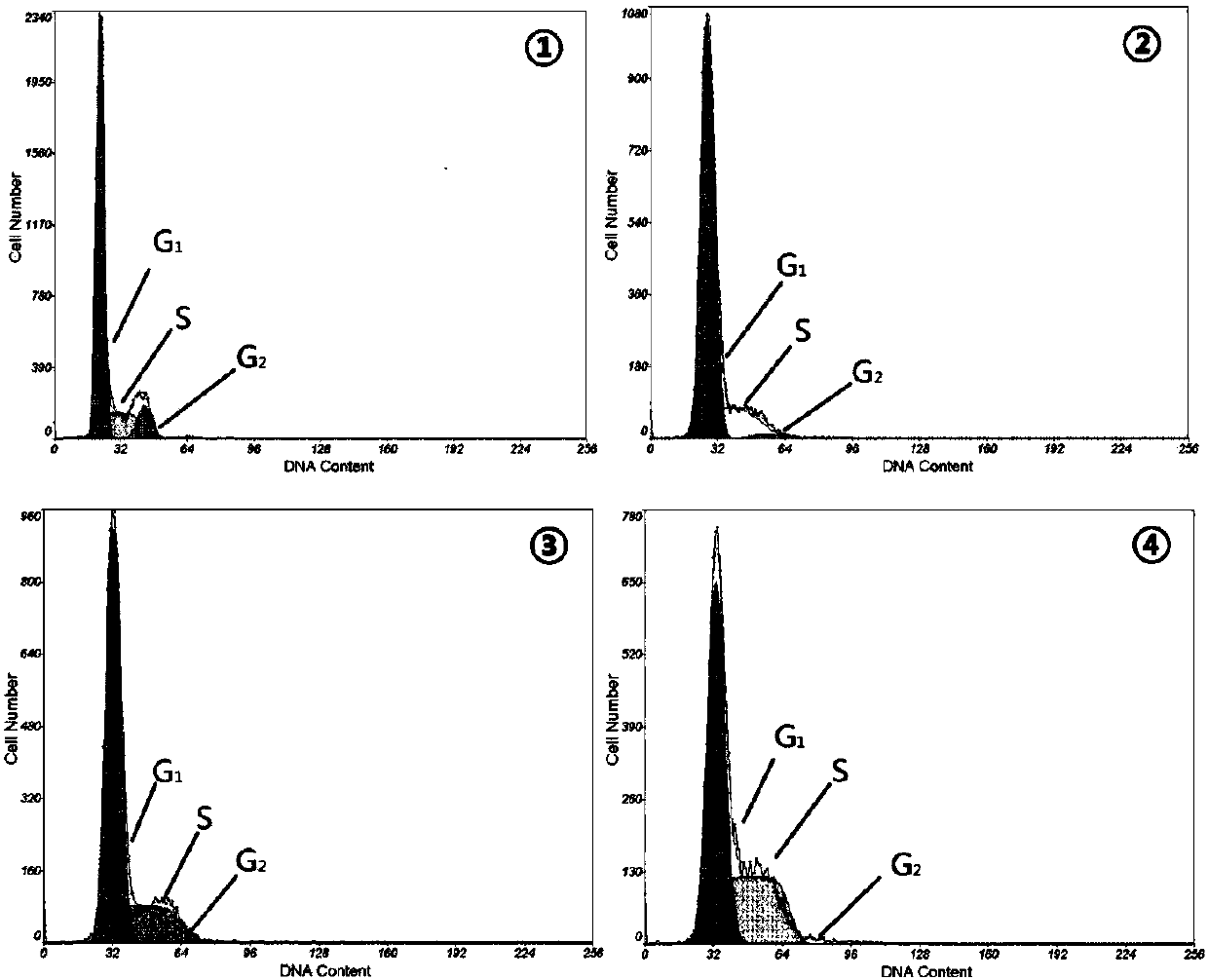
[0053] 2.1 Detection of cell cycle by flow cytometry Take the cells in the logarithmic growth phase, digest with trypsin, resuspend into a single cell suspension, and inoculate in a 6-well plate (the number of cells is 5×10 5 / hole). Cells were collected after 24 h of exposure to different concentrations of huanggui, and washed twice with PBS. Fix with 75% ethanol overnight at 4°C. PI was added to each sample (final mass concentration was 10 mg L -1 ) 10 µL, RNaseA (final mass concentration is 50 mg·L -1 ) 50 µL and PBS 340 µL, mix well, incubate at 37°C in the dark for 30 min, and measure the fluorescence intensity by flow cytometry.
[0054] Results After the cells were treated with huanggui for 24 h, most of the cells were arrested in the S phase, while the cells in the G phase decreased or even lost. Huanggui 0.6, 1.2, 2.4 mg·L -1 The S phase cells in the treatment group increased from 19.97% in t...
Embodiment 3
[0055] Example 3 Effect of Huangguisu on the Apoptosis Rate of MDA-MB-231 Cells
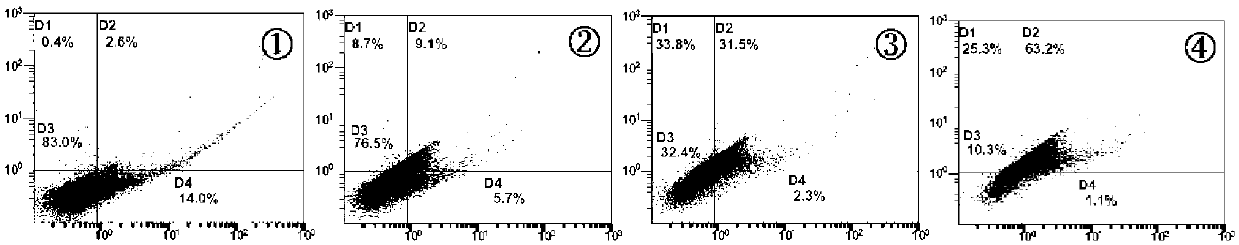
[0056] 3.1 TUNEL method for detection of apoptosis in tumor tissue The tumor tissue specimens were embedded in paraffin, and the slice thickness was 6 μm. The TUNEL test was carried out according to the instructions of the kit. In the TUNEL-stained sections, the nuclei had yellow-brown granules or patches were apoptotic cells. Five slices were made in each group, and five high-power fields (×400) were randomly selected for each slice, and the percentage of positive cells in 1000 cells was calculated, that is, the apoptosis index.
[0057] Data processing and results The median inhibitory concentration (IC) of cells was calculated by medium-effect linear regression 50 ); for other measurement data ±S means, SPSS 13.0 statistical analysis, comparing the significance of the difference between the two groups. The result is as image 3 As shown, after the cells were treated with huanggui for 24 h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com