Application of heteroatom-doped porous carbon coated cuprous phosphide composite catalyst
A technology of cuprous phosphide and porous carbon, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of less research, achieve good mass transfer channels, improve electrocatalytic activity, The effect of a large contact area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
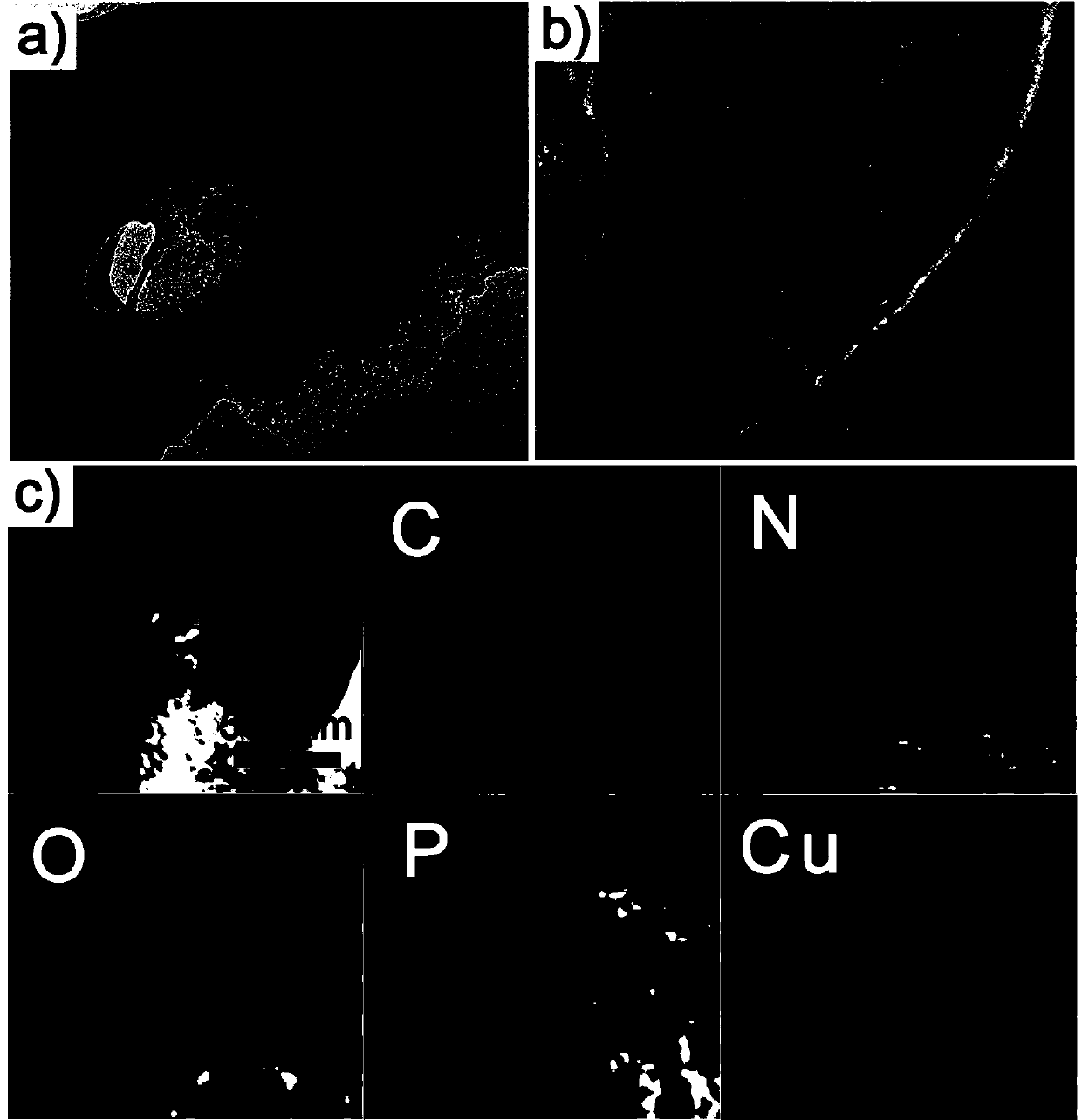
[0036] Example 1: Synthesis of heteroatom-doped porous carbon material coated cuprous phosphide composite catalyst.
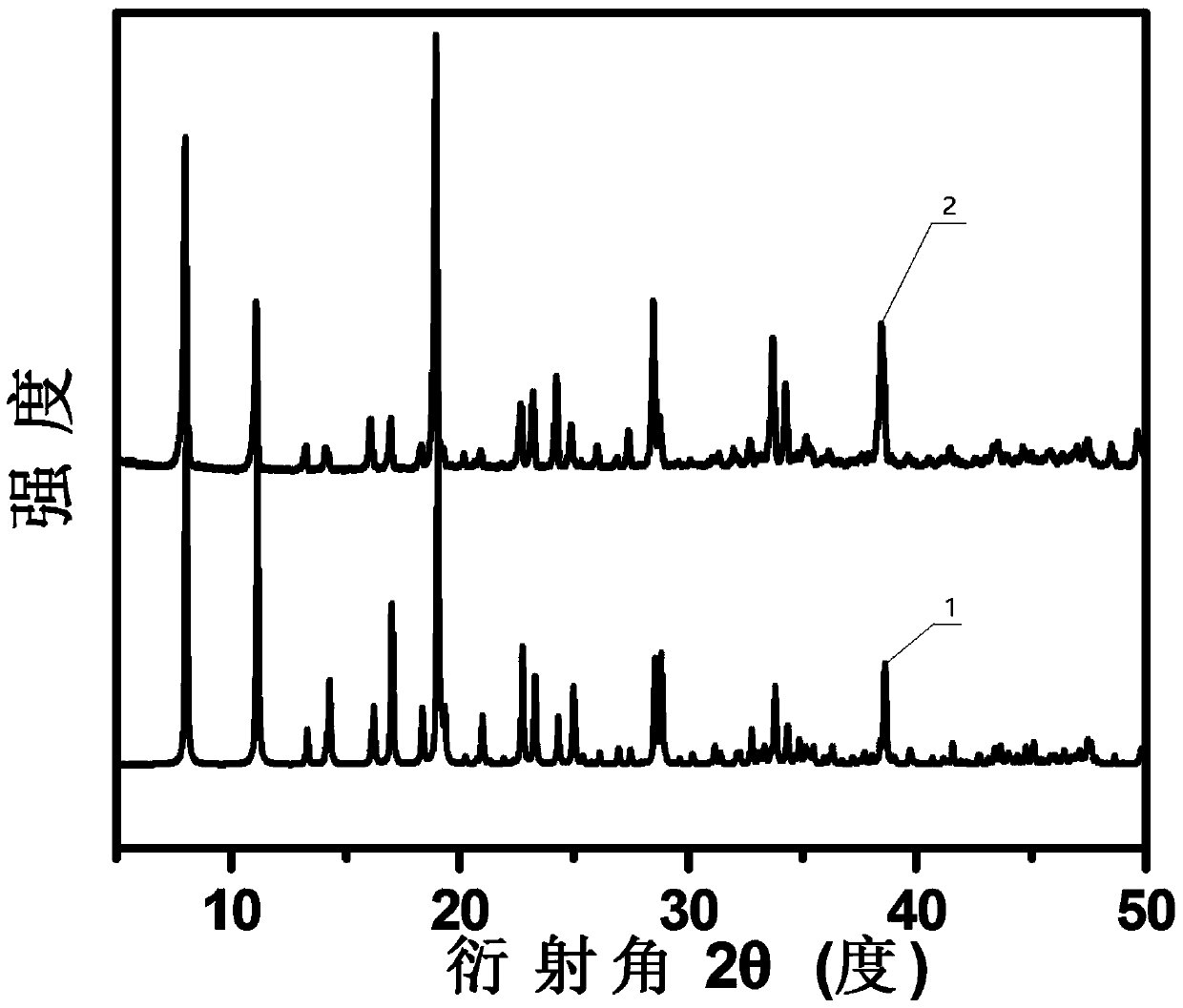
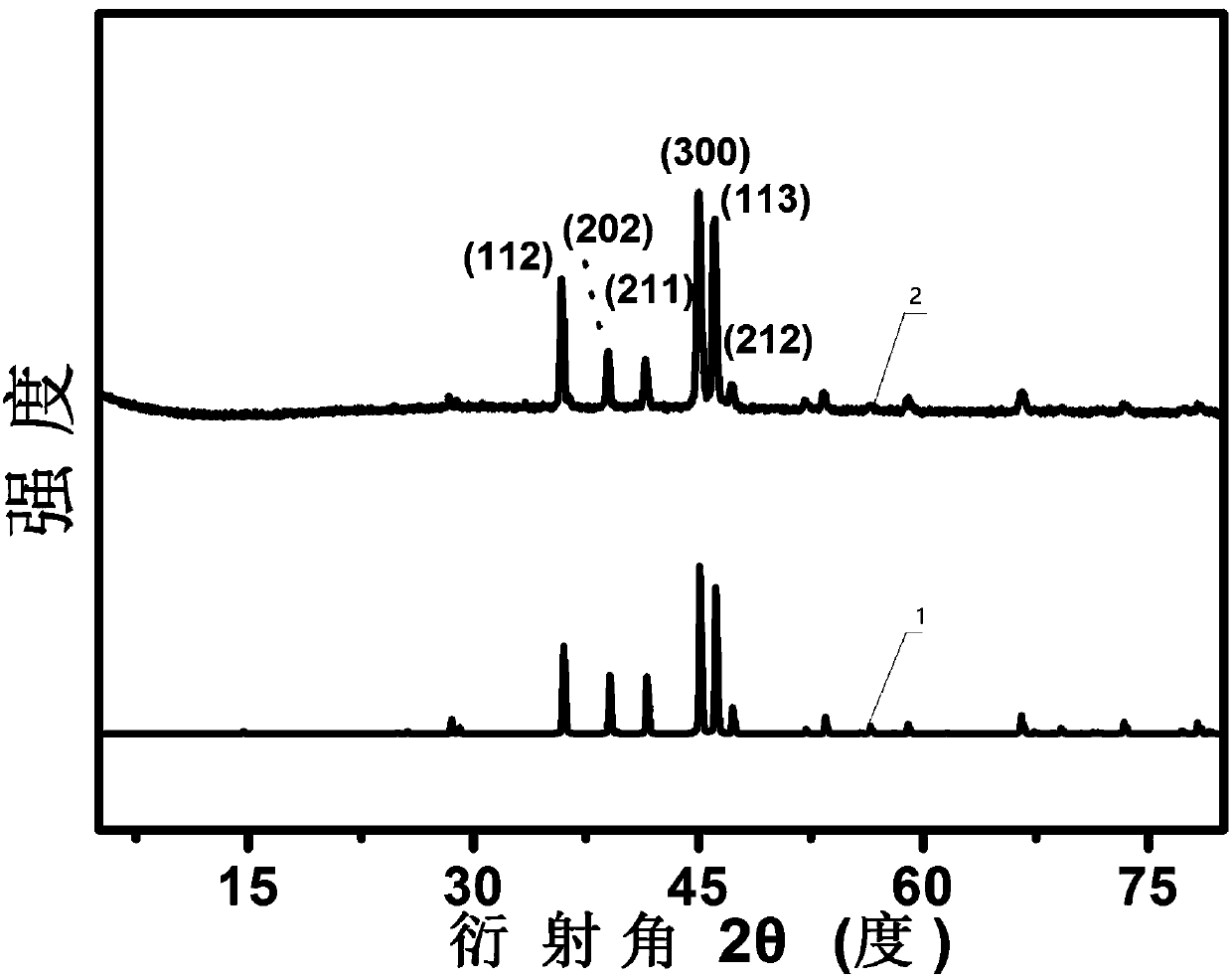
[0037] (1) Dissolve 0.52 g of copper nitrate, 0.55 g of hydroxyethylidene diphosphonic acid and 0.16 g of pyrazine in 20 mL of deionized water, and add sodium hydroxide to the above solution to adjust the pH of the system to 3. Then stirred and refluxed at 140° C. for 6 h to obtain a blue turbid solution. After filtering, washing with water until the filtrate is neutral, the precursor (Cu-NPMOF) blue solid powder is obtained after vacuum drying.
[0038] (2) Put 200 mg of the blue solid powder obtained in step (1) into a quartz boat, place the quartz boat in a tube furnace, and first pass nitrogen gas for 30 minutes to remove the air in the furnace, and then under a nitrogen atmosphere, the tube furnace starts The temperature was raised to 650°C at a rate of 10°C / min, and calcined at a constant temperature of 650°C for 4h. Naturally cooled to room temperature...
Embodiment 2
[0040] Example 2: The performance test of the heteroatom-doped porous carbon-coated cuprous phosphide composite material prepared by the present invention as an electrocatalyst.
[0041] 2mg of the present invention Cu 3 The P@NPPC catalyst was added to a mixed solution of 400uL ethanol and 80uL deionized water, 20uL of 5% Nafion solution by mass percentage was added, and a uniformly dispersed catalyst slurry was obtained after ultrasonic dispersion for 30min. 10 uL of the slurry was drop coated on the rotating disk electrode and dried at room temperature. The electrocatalytic performance test adopts a three-electrode system, with silver-silver chloride (Ag / AgCl) electrode as the reference electrode, platinum wire as the auxiliary electrode, 0.1mol L -1 Potassium hydroxide aqueous solution is the electrolytic solution. The test instrument is a constant potential / current meter of Wavedriver10 from Pine Company, and an MSR rotating disk electrode device.
[0042] like Figur...
Embodiment 3
[0046] Example 3: Application of the heteroatom-doped porous carbon-coated cuprous phosphide composite catalyst prepared in the present invention in zinc-air batteries.
[0047] combine Figure 13 Schematic diagram of the zinc-air battery setup used for testing. The negative pole is a zinc plate, and the positive pole is nickel foam loaded with the catalyst prepared in the present invention. The electrolyte is 6mol L -1 Potassium hydroxide aqueous solution, the positive and negative electrodes are separated by a diaphragm, the side of the positive electrode in contact with the air is a gas diffusion layer, and the gas diffusion layer is made of conductive carbon black and polytetrafluoroethylene.
[0048] Figure 14 Polarized discharge curves and corresponding power density curves for Zn-air battery devices assembled with the as-prepared catalysts at current densities of 10 and 100 mA cm -2 , the battery voltages are 1.27 and 0.88V respectively, and the maximum power densi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com